Summary
Antlers are bony structures, distinct from horns, unique to deer and carried by the males of most species – exceptions include the Chinese water deer (Hydropotes inermis) and the Musk deer (Moschus sp.), neither of which possess antlers, and the reindeer or caribou (Rangifer tarandus) in which both sexes grow antlers.
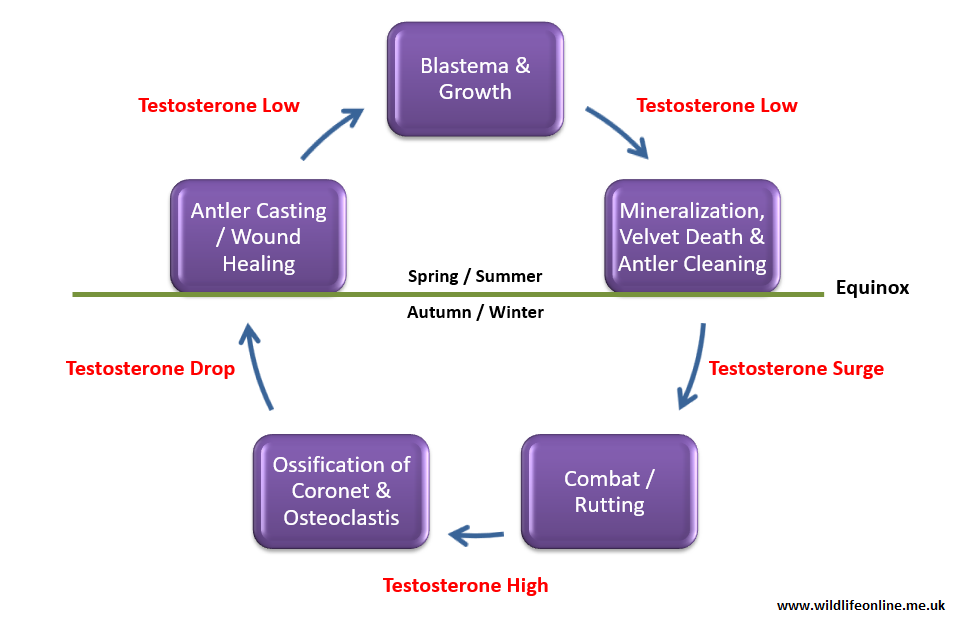
Antlers grow from pedicles, permanent outgrowths of the skull’s frontal bone, and are covered by a layer of hairy skin called velvet during their development. An increase in circulating blood testosterone leads to a shedding of the velvet and reveals the antlers in time for their use during the breeding season. Thus, in most species, antlers develop while the testes are in a regressed state – the exception is the Reeves’ muntjac (Muntiacus reevesi), which remain fertile throughout the year but grow and cast antlers cyclically.
Males use antlers when battling with each other for access to females during the rut. Female reindeer use their antlers, which are smaller and less elaborate than those of mature males, to compete with males for food and defend their calves during the winter, antlers allow them to compete for food during winter and offer some protection to their calves. Once the breeding season is over, a drop in circulating testosterone leads to the antlers being cast and the cycle begins again.
The antler cycle (growth, velvet shedding and casting) is highly correlated with the season; specifically with photoperiod. Antler complexity increases with age until the animal reaches senescence and the size/weight of the antlers is closely correlated with body size. Older males cast their antlers before younger stags and this may be at least partly because it takes longer to grow larger, more complex sets. Antler size/weight is related to habitat and particularly food availability, although genetics seems to be important when considering antler morphology.
Failure to grow pedicles prevents antler growth, while hormonal imbalances (e.g. caused by testicular mutilation or castration) can disrupt the development and lead to the production of deformed antlers. There is some suggestion that parasite load may also be related to certain antler deformities.
The Details
Eighteenth century French naturalist Georges Louis Leclerc de Buffon insisted that antlers were made of wood. Quite how de Buffon considered a wooden structure became attached to a deer’s head is something of a mystery, but cast antlers do possess a distinct similarity to wood, in terms of texture and weight. Despite some early misinterpretations, it was the French zoologist Georges Cuvier who established the true nature of antlers in 1817. Put simply, antlers are bony appendages grown by, indeed unique to, deer – 36 of the 40 known species possess them.

The first crucial distinction to make is the difference between antlers and horns, the appendages possessed by many other ungulate species. Some older texts use ‘antler’ and ‘horn’ interchangeably, but there are significant differences between the two structures. Horns and antlers start life in similar ways, as bony outgrowths of the frontal (forehead) bones. In horns, however, this bony core becomes surrounded by a ‘sleeve’ of fibrous material; it’s composed of structural proteins, primarily keratin (the strong protein found in your hair and fingernails). The sleeve continues to grow throughout the animal’s life, new material produced by modified cells at the base, such that the horn becomes progressively longer, and usually twisted, as the animal ages. (Bovid and giraffe horns form in a different way to those of antelope, but they end up the same – a keratinous sheath surrounding a bone core.) Antlers, by contrast, are replaced periodically and do not grow directly from the skull.
Osteology 101: bone biology and pedicles
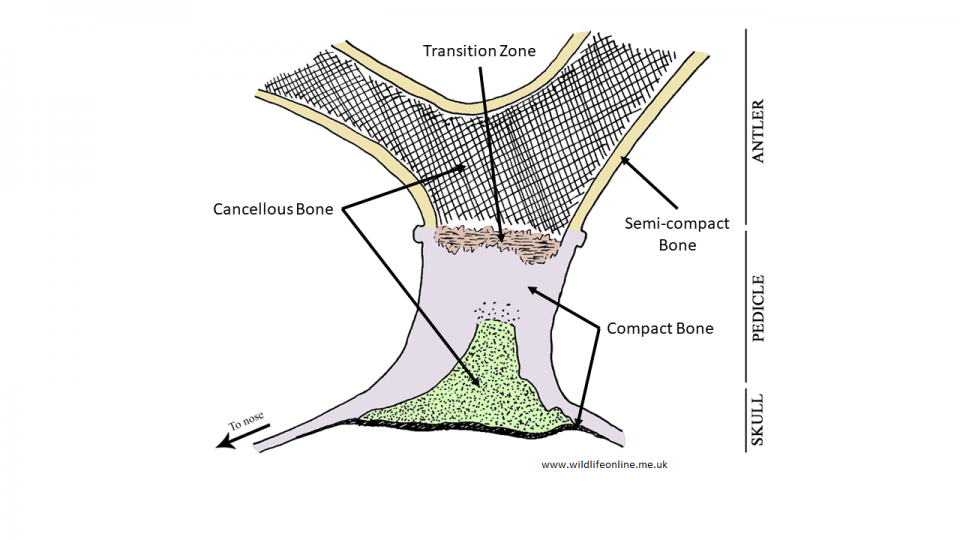
Before we consider the process of antler development, we need to cover a little bone biology. Histologists typically class bone as one of two types. There is the mature bone that forms the skeleton of adult mammals, and the immature bone that is produced during embryonic development and when repairing fractures. Mature bone can be either the hard, compact cortical bone that has very few air spaces (a porosity of 5% to 30%) and makes up about 80% of the adult human skeleton, or trabecular bone, which is sometimes called “cancellous” or “spongy” bone owing to its network of rods and plates that gives it a porosity of up to 90%. Cortical bone forms the outer layer (or ‘shaft’) of a bone, while trabecular bone is the filling. In deer, as in most other mammals, the forehead consists of paired frontal bones that are composed largely of trabecular bone.
In a 1973 paper to the Journal of Embryological and Experimental Morphology, Gerald Lincoln demonstrated that male Red deer (Cervus elaphus) foetuses receive a surge of testosterone just prior to the sexual differentiation that occurs at about 42 days old (i.e. up until this surge the foetus is neither male nor female). It seems that once this differentiation has happened an ‘outgrowth’ starts to form on each plate just in front of the eye sockets. These 'bumps' are composed of trabecular bone and can thus be considered extensions of the frontal bones and are called pedicles (from the Latin meaning ‘little foot’). Lincoln observed that pedicles appeared on Red deer stags about 60 days into development, during the second half of the gestation, although subsequent growth of the skull caused them to become less obvious as development progressed and they were imperceptible by about 115 days.
Lincoln also recorded what he described as “opaque, slightly raised areas in the position of the antler pedicle” on hind foetuses; but these failed to expand in the same way they did in stags, presumably because the females weren’t subjected to the same testosterone surge. In many deer, it is a while after birth before the pedicles are visible and, in his comprehensive review of the subject for Mammal Review in 1975, the late deer biologist Donald Chapman wrote that:
“... in Fallow and Red deer in southern England, pedicles could not be felt in newly-born male animals and the skulls of many six-month-old male animals showed no sign of pedicle development ...”

It appears that, as with so much of a deer’s development, the quality of the habitat strongly influences when the pedicles begin to grow and by when they have completed their development. Several authors have noted, for example, that Red deer in the high quality environment of a deer park can have pedicles by three months old, while those on the impoverished hillsides of Scotland may not have functional pedicles until their second or third year. On average, Red stags in the wild will begin antler development at about 10 months old. Fallow (Dama dama) and Sika (Cervus nippon) deer living in favourable conditions tend to begin antler development at 11 to 15 months old.
Roe (Capreolus capreolus) start growing antlers much sooner. In his 1995 book The Roe Deer, Richard Prior notes that Roe bucks have palpable pedicles by three months old; they can be seen with the naked eye come September (about four months old) and, if well fed, the buck often has small antlers (called ‘buttons’) by January at the age of about eight months. Earlier authors have recorded remarkably precocious antler development in Roe deer, with buttons complete by four months old! Muntjac deer (Muntiacus reevesi) have observable pedicles by about five months old, with antlers grown from May to September.
In his Mammal Review paper, Chapman noted that, as a general rule of thumb, the telemetacarpalian deer (e.g. Roe – see Deer Taxonomy) begin antler development in the autumn of their first year, while the plesiometacarpalian species (e.g. Red, Sika and Fallow) don’t start until early in their second year. Regardless of when development begins, the first antlers are usually simple, un-branched spikes; only in exceptionally well-fed animals are first antlers sometimes branched. These first antlers grow as extensions of the pedicles and consequently lack the elaborate base, called the coronet or burr, that mature antlers have.
Growth and development
The precise biomechanics of pedicle formation, ‘growth zones’ and antler development are outside the scope of this article, but readers interested in a technical appraisal are referred to the papers listed at the end of this section – that which follows is a summary based on those sources.
Laying the foundations: Pedicle development
The basic development can be divided into two stages: the initial formation of the pedicle and the growth of the antler on top of it. Histological studies have found that it is the membrane that surrounds the pedicle, the periosteum as it is known, that is crucial in allowing the antler to form.
In a series of eloquent, if somewhat Frankensteinian by modern standards, experiments German anatomists Hermann Hartwig and Josef Schrudde demonstrated that transplanting periosteum of a Roe deer kid to its metacarpus resulted in an antler being grown on the deer’s foreleg. Several years after these studies, Richard Goss at Brown University in Rhode Island named this tissue ‘antlerogenic periosteum’ (abbreviated to AP), which roughly translates to ‘antler-producing bone cover’.
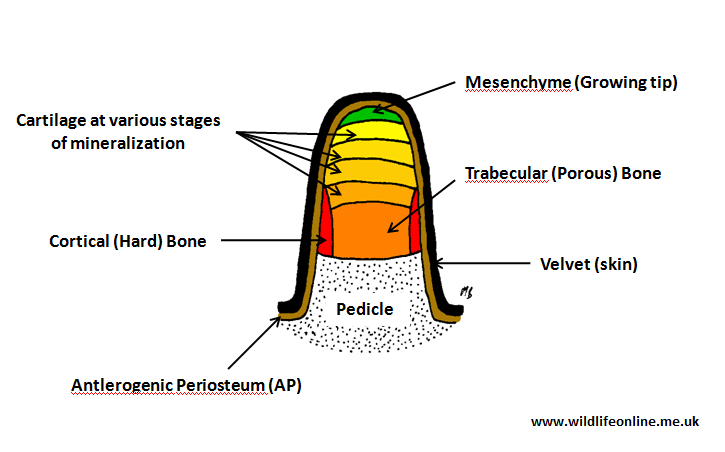
The pedicle begins life as a small section of AP tissue; the cells are spurred into differentiation (i.e. turning into antler[bone]-forming cells called osteoblasts) by a rise in the levels of testosterone in the blood. As the cells multiply and the area grows, minerals (predominantly calcium and phosphorous) are deposited through a process known as mineralization. Mineralization is one of the steps in the process of turning a tissue into bone; a process called ossification. In a brief paper on Red deer to the Journal of Zoology in 1986, Norma Chapman described how, as the antler grows, mineralization advances as a discrete band about two to four centimetres (about 1.5 in.) behind the growing apex (tip).
The pedicle is surrounded by a dense hairy skin, modified from that on the scalp, called velvet. (In Roe deer, this skin is covered with longer hairs than found in other species; presumably an adaption to growing antlers during the winter.) The pedicle remains a permanent feature of the animal’s skull, although it appears to vary in width throughout the deer’s life; growing until the deer reaches senescence, at which point it begins shrinking, and with some suggestion that one pedicle may remain larger than the other.
It appears, incidentally, that the pedicle enforces some degree of polarity on the antler and, in 1991, Richard Goss found that if you cut a disc of the AP, rotate it 180 degrees, and put it back in the same place the antler grows back-to-front. Once the pedicle reaches a ‘threshold size’ (this seems to correspond to a body weight of about 30kg/66 lbs in Red deer) additional bone formation begins at its tip – this is the start of the antler’s growth.
Antler growth
The antler starts life as a small lump of mesenchymal tissue (i.e. a loosely connected lump of unspecialized cells) at the tip of the pedicle that we call a blastema. The blastema is the site of active mitosis (cell division) within the antler; cartilage is laid down that will later be mineralized and ultimately ossified. This setup means that, unlike horns, antlers grow from their tip, not from their base. This was demonstrated during the 1950s in an elegant experiment that saw Herbert Bruhin insert a screw 3.5cm (just under 1.5 inches) from the antler base and another one 1.5cm (just over half-inch) from the antler tip of his subjects. Eleven weeks later, the first screw was still 3.5cm from the base, but the second screw was now 5.5cm (just over 2 in.) from the tip.

There are two main processes going on under the velvet as the antler grows: cartilage in the core is ossified to trabecular bone, a process called endochondral ossification, while the membranes surrounding the core get turned directly to cortical bone in a process known as intramembranous ossification. In other words, the antler grows in height as more cartilage is produced in the core and turned to bone, and it increases in thickness as more compact bone is laid down around the shaft.

While the antler is growing, it needs nourishment in the form of minerals and oxygen supplied by branches of the superficial temporal artery (STA). As Norma Chapman noted in her review, the STA of Fallow, Sika and Red deer branches into about a dozen smaller arteries, each with narrow interiors and thick walls, which ascend the antler and surrounding velvet. Chapman goes on to say: “The flow of blood through the velvet is probably both copious and rapid judging from the warmth of the growing antler tips.”

The big reveal: Shedding velvet

As the breeding season approaches, levels of testosterone in the deer’s blood start to rise and this triggers substantial ossification at the coronet, leading to a restriction of blood flow and a ligation (‘tying off’) of the velvet. The connection between rising testosterone and velvet shedding was established by scientists in the 1970s, who found that castrated stags grew normal antlers but failed to shed the velvet. When the blood supply to the velvet is shutdown the tissue dies and begins to dry up and fall off – at this stage the deer is said to be “in tatters”.
Deer are often seen thrashing their antlers in undergrowth, on bushes and trees in a bid to remove the velvet in a process known as cleaning or polishing. (The decaying tissue may attract flies that land on the antler in such numbers as to turn the it black.) When the velvet first peels, the antlers are white bone, but thrashing and rubbing against vegetation and soil stains them, giving a rustic colour anywhere from black, through most shades of brown to grey. A stag or buck with cleaned antlers is often said to be in the misnomer of “hard horn”.
Once the velvet has been shed the antler is considered ‘dead bone’, because it is unable to grow any further or repair any breakages. As deer biologist Richard Goss pointed out in 1992, however, “nowhere else in nature is dead bone tolerated in an animal, in this case from three to nine months, depending on species”. This got Hans Rolf and Alfred Enderle at the University of Goettingen in Germany thinking about whether the bone really was dead.
In a fascinating paper to The Anatomical Record in 2002, they presented their study on the vascularisation of Fallow deer antlers. Rolf and Enderle found that even after the velvet had been shed the antlers were still being fed by a series of capillaries and vessels from the pedicle. In other words, the antlers had a functioning vascular system that kept them moist; they were living bones. Indeed, it appears that final mineralization of the coronet may not happen until only a couple of weeks before the antler is cast, at which point the bone cells effectively starve and the antler ‘dies’. Indeed, a study of Roe deer antlers in the late 1980s suggested that mineralization may continue well into the rutting season, while data from Fallow antlers imply that some remodelling of bone may occur even after the antler is fully mineralized.
Of course, the discovery that the antler is actually a living bone does not mean that it is also a sensitive bone. The developing antler gets its nerve supply from branches of the trigeminal nerve, the nerve that also serves the ears and eyes, but this connection appears to die back with the velvet and there is no evidence to suggest that the antler retains a nervous connection once the velvet has been shed; breakage of the antler certainly doesn’t appear to cause the deer pain.
Casting and regrowth

The high levels of circulating testosterone causes the antlers to be retained for several months; throughout the breeding season and well into the following year in many species. The final ossification of the coronet and ultimately the shedding (known as casting) of the antlers, is triggered by a drop in testosterone in spring. Histologically, it appears that just prior to casting, a zone forms at the top of the pedicle, often betrayed by a swelling of the skin at the base of the antler, where the bone is broken down by osteoclast (literally ‘bone breaker’) cells that appear directly below the burr or on top of the pedicles – this process is called osteoclastic resorption.
This destruction of bone leads to a weakening of the coronet-pedicle junction; the antler loosens and eventually falls off. In his 1992 opus, The Whitehead Encyclopedia of Deer, G. Kenneth Whitehead wrote that:
“The loosening of an antler, prior to shedding, would appear to be very sudden, and at the critical moment final separation of the antler from the pedicle may be caused by the head being jarred on landing following a jump over an obstacle.”
Indeed, several authors have noted how antlers close to being cast don’t wobble, as our teeth do before we lose them, so osteoclastis presumably occurs rapidly. It appears that the osteoclastis is considerable and, in his 1992 review on the biology of antlers, Gerald Lincoln noted how, if casting is prevented by a hormone injection, the ‘die-back’ of bone progresses down the pedicle into the skull and can be fatal.

Deer are often said to have cast their antlers simultaneously if both are lost within 24 hours; when one antler is lost more than 24 hours after the first it is referred to as asynchronous casting. Despite the terminology, the antlers are very seldom cast in a truly simultaneous manner and they can be shed anywhere from a few minutes to several hours, even days, apart. Once an antler has been cast, the deer is left with an open wound on the top of the pedicle. Interestingly, there is generally little blood loss and remarkably few cases of reported infection.
The wound healing process on the pedicle is exceptionally rapid and within hours of casting the pedicle will have been covered by a scab, and new velvet will be growing over it. (Technically, it is an ‘antler regeneration blastema’ that forms from connective tissue that survives osteoclastis, rather than a true scar, but the end result is the same.) Histological studies by German anatomist Uwe Kierdorf at the University of Hildesheim and others have demonstrated that, under this newly-formed skin there is a brief period during which more bone is broken down, giving the pedicle a smooth surface, before bone growth resumes and the antler regeneration process starts over.

Until recently we thought a burr was a requirement for antlers to be cast and that early examples of antlers, which date back to the early Miocene (21 mya) and lacked these burrs were permanent structures called ‘proto-antlers’. A more recently analysis by Nicola Heckeberg at the Ludwig Maximilian University in Munich suggests, however, that a burr is not a prerequisite to shed antlers. In a paper to the Journal of Morphology in 2017, Heckeberg provides a detailed analysis of the antlers of several fossil species and argues that even early ‘burr-less’ antlers were shed and that, although a typical feature of the antlers we see today, is probably actually evolved as a by-product of repeated bone building and intensive ossification.
Based on Heckeberg’s findings, early Miocene species such as Procervulus from Germany and Acteocemas and Ligeromeryx from France could shed their antlers, suggesting that they now represent the oldest known species of true deer. Prior to this, the middle Miocene (ca. 13 mya) species Euprox minimus was considered the earliest antlered deer, being the oldest species known to have burrs.
What’s in a name?
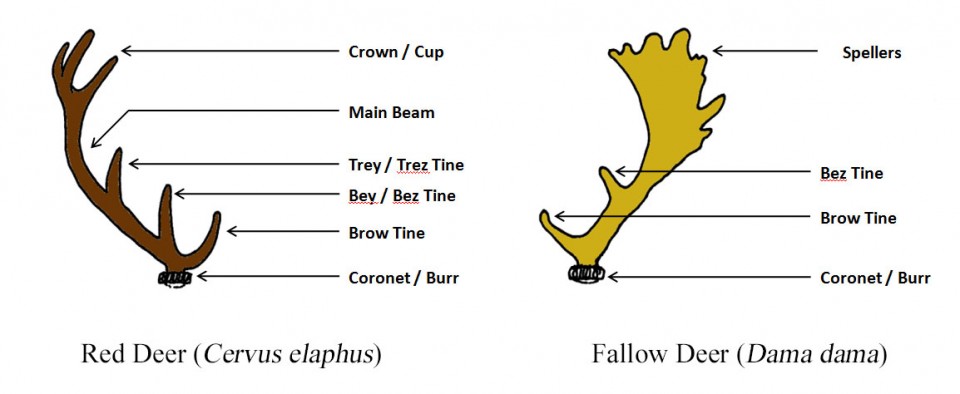
A mature Red stag may well have 12 to 15 branches, called tines or points, to his antlers and stags are often named according to the number of these points. Deer with their first set of short, simple, unbranched antlers (i.e. at two years old) are referred to as prickets (Fallow) or brockets (Red). Over subsequent years, the antlers should become progressively larger and branched up until the stag is about 10 years old, after which the number of tines starts to decline. A Red deer with 12 points (six per antler) is called a Royal stag, while 14 points make an Imperial stag and an animal with 16 points or more is referred to as a Monarch.
In his article for South Coast Today, a Massachusetts news and current affairs website, outdoor writer Marc Folco described how hunters speak in terms of “pointers”. Folco explains that a deer with five tines each side is a five-pointer, while one with six either side is a six-pointer. In cases where the antlers are asymmetrical (i.e. different number of tines each side), the two values are given separated by an “X” – thus, a deer with six tines on one antler and five on the other is a “6 X 5”, rather than an 11-pointer. In Fallow bucks, the palmation extends with subsequent antler sets as do the number of points, called spellers in this species.
| Red Deer (Cervus elaphus) | Fallow Deer (Dama dama) |
|---|---|
| Yearling = Calf | Yearling = Fawn |
| Second Year = Brocket | Second Year = Pricket |
| Third Year = Spayad | Third Year = Sorel |
| Fourth Year = Staggard | Fourth Year = Sore |
| Fifth Year = Stag | Fifth Year = Bare Buck |
| Sixth Year = Hart | Sixth Year = Buck |
| Seventh+ Year = Great Hart | Seventh+ Year = Great/Master Buck |
That time of the month

It was Greek philosopher Aristotle who first noticed that the genitals had an important connection to the development of antlers and, around 350 BC, he wrote in his Historia Animālium that:
“If stags be mutilated, when, by reason of their age, they have as yet no horns, they never grow horns at all; if they be mutilated when they have horns, the horns remain unchanged in size and the animal does not lose them.”
It would be several centuries before Aristotle’s musings were confirmed experimentally when, in 1913, two German anatomists established that castration of a deer with cleaned antlers led to casting and growth of new antlers from which the velvet is never shed. Subsequent experimentation by Richard Goss found that both androgens (testosterone) and oestrogens (oestradiol) could prevent old antlers from being shed, inhibit the growth of new antlers and cause the velvet to shed prematurely from growing antlers.
It has also been shown that castration of a stag growing antlers prevents the antlers from becoming completely calcified and the velvet from being shed; the result is that the antlers may continue to grow and lead to what Donald Chapman described as “antler monstrosities”, including the benign tumours that Goss called ‘antleromas’. Studies by various authors, especially the Red Deer Research Group on Rum and Richard Goss, during the 1970s and 80s provided further confirmation that testosterone was the regulator; it was found that fitting castrated stags with testosterone implants allowed them to resume a normal antler cycle, while testosterone injections initiated pedicle development in females.
Curiously, reindeer seem to be the exception and studies by Swedish biologists Sven Skjenneberg and Lars Slagsvold have found that castrated adult male reindeer continue to replace their antlers annually. We’re not sure why, yet. Similarly, we know that many of the genes involved in antler growth are recruited from neural tissue, such as the brain, and this perhaps explains why Anthony Bubenik and colleagues found electrical stimulation of antler nerves or antler periosteum of white-tailed deer (Odocoileus virginianus) increased antler length by just over 70% and weight by 40%. Thus, it seems that neural tissue plays an important role in antler growth and regeneration, but the specifics are unknown at present.
Shedding light: the importance of photoperiod
The question of what regulates testosterone is less straight forward to answer, although for temperate deer at least (equatorial deer appear to be a special case, as will become apparent) light is critically important. More specifically, it is the number of hours of light and darkness that a deer receives in a 24 hour period. This is referred to as the photoperiod and expressed as a light:dark ratio. A ratio of 12L:12D would, for example, mean 12 hours of light followed by 12 hours of darkness.

Temperate deer are highly seasonal animals and their biological rhythms are heavily influenced by the changing daylength. Melatonin, the so-called ‘hormone of darkness’, is produced by the pineal gland in response to darkness and the more hours of darkness the animal is exposed to, the more melatonin is produced. There are some ambiguous data about the role of melatonin on sex hormones in mammals, but in short-day breeders (i.e. animals that breed during the autumn or winter) it seems that gonadal functions are activated by an increase in melatonin. Thus, an increase in melatonin indirectly stimulates the testes to increase their testosterone production.
The increase in circulating testosterone then terminates bone formation in the antler and triggers the death of the velvet. Indeed, biologists at Aberdeen’s Rowett Research Institute reported, in a paper to the Journal of Reproduction and Fertility during 1986, that melatonin is a key hormone in regulating the antler cycle; they demonstrated that velvet shedding and rutting could be induced up to five weeks early in a stag given food pellets containing the hormone.
The importance of photoperiod on antler cycle has been recognised for more than half a century when, in 1954, Polish biologist Zbigniew Jaczewski demonstrated that Red deer could produce two sets of antlers in a single year if exposed to a ‘sped up’ photoperiod. Despite these initial results the ways in which photoperiod regulated antler development was, and to an extent still is, poorly understood.
A series of classical experiments on Sika deer (Cervus nippon) conducted by Richard Goss at Brown University in Rhode Island, however, improved our understanding. During the seven year study, captive deer were held under the artificial conditions of Brown University’s Animal Care Facility, where the photoperiod and temperature were strictly controlled – the idea was to expose the deer to various combinations of light and dark and see what effect it had on their antler cycle. Goss found that, if the photoperiod was reversed (i.e. the deer thought it was winter when it was actually summer) they cast and re-grew their antlers six months out-of-sync with the outside world. It was also possible to corroborate and expand upon Jaczewski’s studies.

Goss demonstrated that, if the photoperiod was reduced to replicate a shorter year, the deer could be made to regenerate up to four sets of (albeit stunted) antlers in a single calendar year. Interestingly, Goss observed that when the photoperiod was set to mimic ‘six years in one’ the deer didn’t produce six sets of antlers, instead they returned to their normal circannual cycle of one set per year. Likewise, when the photoperiod was increased to one year last for two, the deer still cast their antlers each calendar year as normal.
Overall, Goss concluded that all the time the deer could determine the photoperiod they could cast and re-grow their antlers accordingly, even if this led to increased or irregular production. If, however, the photoperiod was one that they couldn’t work out they simply reverted to their usual circannual rhythm of casting and renewal. In other words, there is a limit to the photoperiod that deer can adapt to.
Equatorial deer, which aren’t exposed to differing photoperiods throughout the year, can be found with antlers at any time of the year. Curiously, however, many still seem to cast annually; so they must have a method of keeping track of time, although we don’t know what that is. Goss’ work shows there’s something peculiar about an equatorial photoperiod (i.e. 12L:12D). His deer cast normally when given 12L but fewer dark hours and vice versa; but when given 12L:12D they failed to cast, and casting was prevented for up to four years in one animal.
At first, Goss thought it might have been the L:D ratio of one that caused it, but exposing the deer to shorter durations of the same ratio (i.e. 5L:5D, 6L:6D, 8L:8D and so on) caused the deer to cast as normal, so there was something special about 12 hours of light followed by 12 hours of darkness that caused disruption to the cycle and a failure to cast.
We still don’t know what happens or how equatorial deer manage to keep track of the year, although there is the suggestion that they are sensitive to how many alternating periods of light and dark they experience and associate a certain number with one year. Indeed, Goss’ work has provided interesting data to suggest that deer may be able to keep track of photoperiods.
By splitting deer into two groups and exposing one to a regular short-day to long-day photoperiod (i.e. 4L:20D to 20L:4D) and the other to the opposite, Goss found that antlers were shed and re-grown in synchrony with every alternative change in day length, regardless of the direction (i.e. lengthening or shortening). In other words, it didn’t matter whether the days got longer or shorter, the important thing was that the deer registered that there had been a switch in the photoperiod and, because they normally experience two shifts per year, they adjusted their cycle to shed every second change. This result is curious, however, given what we know about the function of hormones in this cycle. Indeed, it’s difficult to see how it could only be the number of shifts in photoperiod that a deer registers as opposed to the amount of light the shifts bring, which would have a direct stimulatory or suppressive impact on melatonin production. Sufficed to say, the jury is still out.
In the end, we have a cycle of bone growth and loss that is under the control of light; light stimulates the production of melatonin, which probably acts on the anterior pituitary stimulating the testis to produce (or stop producing) the testosterone that regulates the deposition of bone within the antler, and the blood supply to the velvet surrounding it. The next step is to address what controls how the antlers look and how large they grow.
Keeping up appearances
For centuries the size of a deer’s antlers has been of interest to humans. In years gone by, Red deer were transported from English parks to the hillsides of Scotland in a bid to improve the quality (body and antler size) of the native stock; this implies an element of supposed genetic control over the development of antlers. Indeed, many have commented that it is possible to identify a stag over successive years by the shape of his antlers; the Red Deer Research Group on Rum have apparently become rather adept at this. The evidence, however, is far from unanimous and, in his book The Roe Deer, Richard Prior wrote:
“A buck’s age, let alone his identity or his value as breeding stock, cannot be judged by his antlers alone.”

It is some of these translocations that have shown us it is not solely genes that determine how large antlers grow; diet is a crucial factor, too. In his Whitehead Encyclopedia of Deer, Kenneth Whitehead described how Scottish hill stags, which are typically rather small-antlered animals, transferred to the superior habitat of a deer park are capable of producing antlers comparable to stags in other high quality habitats, such as deciduous woodlands. Indeed, Prior stated that it was habitat, rather than bloodline, that had overriding influence on the antler size of Roe deer. In his A Life for Deer, vet John Fletcher made much the same point when he said:
“… undoubtedly the limiting factor in the productivity of Highland red deer is very rarely the genetics of the deer but rather the environment: food and shelter”.
There are other population studies, largely on Rum, suggesting that the average antler size declines with increasing population density – more deer means more competition for food such each animal is in poorer condition, thus producing smaller antlers. The Rum biologists have also found that the weather and early life-history of the deer can affect the length of their first antler set – as may be expected, light calves born late in the year and growing up in bad weather (mainly low temperatures) developed smaller antlers than heavier calves born during good conditions.
Despite a considerable body of evidence implicating habitat quality in regulating antler size, this doesn’t mean that genetics are unimportant. Ultimately, all structures in the body are built according to the ‘blueprint’ laid out by the genome and it’s well established that there is a species-specific pattern to antlers (i.e. you can tell the species by looking at its antlers), which is presumably encoded in their genes. Indeed, we now have several studies showing that there is a degree of heritability in both size and, perhaps more importantly, shape of the antlers; the researchers on Rum estimate that antler size is roughly 20% heritable. Overall, the data suggest that, where food isn’t limited and hormonal aberrations (accidental castration, etc.) are absent, genetics play a fundamental role in determining how large and into what shape antlers will grow.
When dietary and hormonal aberrations are encountered, these can override the impact of the genes because such secondary sexual characteristics (i.e. features that aren’t necessary for the physical act of reproduction) generally have low growth priorities. Despite the antlers growing larger (taller and thicker) as the deer ages, up to senescence, there does not appear to be a correlation between the age of a deer and the number of antler tines.

The understanding that antler size and complexity increases throughout the stag or buck’s lifetime explains why it should be necessary to cast the antlers they have invested so much energy growing. The sensitive velvet skin is necessary for the antler to grow, but must be removed before it can be used in combat. Removing the velvet, however, effectively ‘kills’ the antler because, despite some bone cell activity as mentioned above, the antler cannot grow or heal once cleaned. Thus, if the antlers are to grow with the deer’s body, it becomes necessary to replace them regularly.
As we shall see, antler size and complexity appear to help females judge how ‘fit’ a male is, as well as potentially letting other males decide whether he’s worth fighting with. If the antlers were never replaced, the male would either spend his entire life with simple spikes that said nothing about his reproductive prowess, or would need a set that were impracticably large for a yearling to carry. All this casting and re-growing, however, doesn’t come without a price.
The cost of antlers
Antlers come in all shapes and sizes, from the small (10cm / 4 in.) simple spikes of the Reeves’ muntjac (Muntiacus reevesi) to the 1.5m (5 ft) organs sported by American wapiti (Cervus canadensis). Moreover, we have seen that antlers are grown quickly. Indeed, they are the fastest growing mammalian tissue; they exhibit a typical sigmoidal growth curve (i.e. start slowly, speed up and then slow down just prior to cleaning), in some species growing by as much as 10 centimetres (4 inches) per day during their peak growth period, and are complete within 12 to 16 weeks. Such rapid growth requires a considerable amount of minerals, namely calcium and phosphorous.
In 1985, Paul Muir and his colleagues at the University of Canterbury in New Zealand calculated that a Red stag producing three kilos (just over 6.5 lbs) of hard antler deposits just over half-a-kilo (19 oz.) of calcium and up to one kilo (just over 2 lbs) of other minerals; during the final ten weeks of development, when growth is at its most rapid, calcium is deposited at a rate of around 5g (1/5 oz.) per day. This corresponds well with values given by Donald Chapman who, in his Mammal Review article, notes that antlers are composed of about 50% minerals (the rest is largely ash and protein) and, of this, 45% is calcium and 19% phosphorous. So, as Chapman points out, for an ‘average’ Red stag producing a pair of antlers weighing around 13kg (just under 3 lbs) in 130 days, this means that the deer must produce an average of 100g (3.5 oz.) of bone per day.

So, how do the deer find such large quantities of bone growing minerals in such a short time? One source is the bone already in the deer’s body; it’s skeleton. Work on several species, including Mule deer (Odocoileus hemionus) in the Rocky Mountains of Colorado and Reindeer kept at Washington State University, has documented a decrease in bone mass during antler growth, caused by osteoporosis or osteoresorption. Minerals appear to be removed from the skeleton, with the ribs and long bones (e.g. the metacarpus and tibia of the legs) yielding a combined decrease in density of almost 60%; the bulk of this resorption seems to occur during the middle of the antler’s growth period. It seems that, where resorption occurs, it is more common in trabecular bone than in cortical bone, probably because the former is more metabolically active.

Obviously, there is a limit to the amount of minerals that can be sequestered from the skeleton before the bone becomes too brittle to support the animal, and it seems unlikely that the complete antler set could be constructed in this way. This is even more apparent when we consider that antlers are allometric organs. In other words, larger deer grow larger and more complex antlers even after correcting for body weight – small bodied deer grow small, simple antlers. Consequently, deer must get some of their minerals from their diet and one very good source is antlers.
Bone-eating is apparently a fairly common behaviour in deer and cast antlers represent a substantial ‘mineral bank’ – I have even come across reports of deer chewing on another’s antlers while they’re still attached! In their 1982 Red Deer: Behaviour and Ecology of Two Sexes, Tim Clutton-Brock, Fiona Guinness and Steve Albon note that both stags and hinds on Rum chewed bones and cast antlers, especially during the spring and early summer when stags are in velvet and females pregnant.
In a fascinating paper to the journal Mammalia during 1985, Cyrille Barrette described the antler-eating behaviour of wild Axis deer (Axis axis) in the Wilpattu National Park, Sri Lanka. Barrette witnessed 102 instances of this “osteophagia” (literally ‘bone eating’) during his two years of fieldwork, with all ages and both sexes indulging – it was, however, the males in velvet that were seen to chew bones most often. Apparently chewing bouts were fairly lengthy, lasting on average nearly 40 minutes if the ‘chewer’ wasn’t disturbed. In some cases the antler was chewed down to the coronet. Describing the chewing behaviour, Barrette wrote:
“In most cases, the deer did not pick up the bone off the ground but only lifted the sharper end, chewing it with molars and premolars in the ‘cigar-like’ manner described by Sutcliffe (1973). The heavier end of the bone rested on the ground and saliva could be seen dripping while the animal worked the bone in its mouth.”
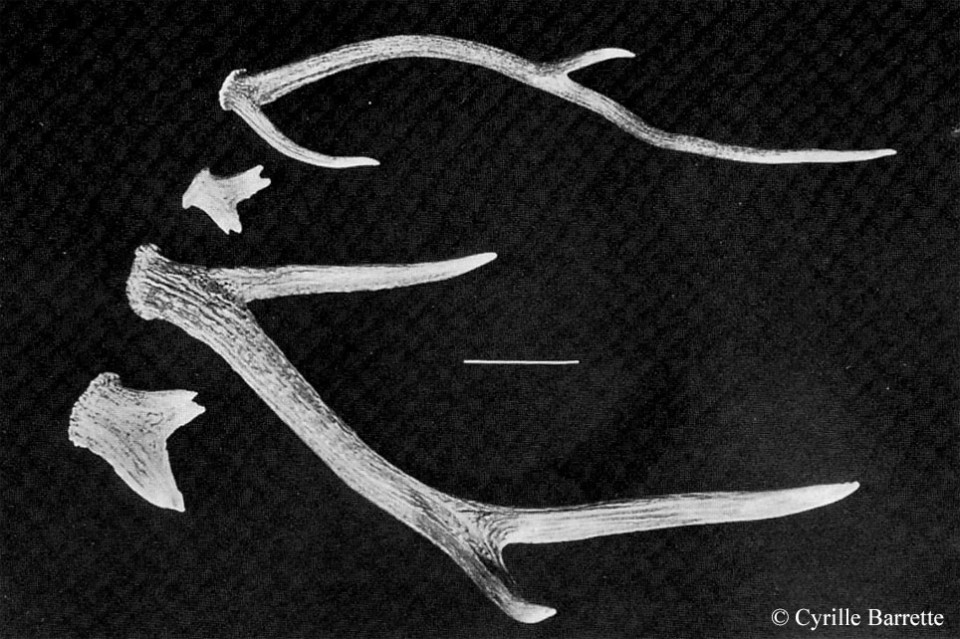
Incidentally, as something of a side-line, the reason that we don’t find ourselves swimming in cast antlers when we go walking in the woods (in fact, they’re pretty scarce) is probably because many are eaten by deer and rodents. Where antlers are found, it’s common to find scrapes and indentations on their surface characteristic of them having been gnawed by rodents. In a brief communication to the journal Science in 1940, former University of Toronto zoologist Alan Coventry told of an American red squirrel (Tamiasciurus hudsonicus) regularly visiting a moose skull outside his cabin on an island in Ontario’s Lake Temagami, to gnaw at the bony projection.
Given the need for replacing antlers each year and the significant metabolic drain involved in doing so, why should the deer go through all this? What do they use these antlers for?
Swiss Army antlers
The function of antlers has long been a subject of debate, with suggestions ranging from the obvious to the bizarre. In 1937, for example, the eminent German zoologist Han Krieg suggested that antlers were a method of removing excessive minerals consumed in the diet. Today, there is little contention over the purpose antlers serve or the reasons for their evolution. Before we look at the currently accepted theory of purpose, let’s take a moment to look at some of the competing ideas.
One thing we can be fairly certain of is that, given the high energetic cost associated with growing antlers, if deer didn’t have a good ‘reason’ for doing so, they almost certainly wouldn’t. Nature is a balancing act, and animals that waste their energy on frivolous organs are lost from the population. Consequently, having antlers must convey an advantage that is genetically heritable. In other words, having antlers must make it more likely (certainly at least as likely) that you’ll survive and reproduce, thereby sending your genes, which contain the instructions for building antlers, into future generations.

In a short paper to the journal Nature during 1968, Bernard Stonehouse at the University of Canterbury suggested -- based on various anatomical observations, including the large number of blood vessels, lack of fat under the skin and branching that provides a large surface area -- that:
“Thermoregulation may thus be the function which primarily determines the form and proportions of antlers, and necessitates their annual renewal”.
In other words, antlers might have evolved to help deer regulate their temperature by dissipating heat, much like the ears of an elephant. The immediate problem with this idea is that generally only males grow antlers; surely if it was a heat loss adaptation females would grow them too? Well, Stonehouse argued that males have a bigger problem losing heat because they put on weight more rapidly and maintain larger fat reserves than females during the summer months. In his review of the function of antlers published in Behaviour during 1982, however, Tim Clutton-Brock suggested that the anatomical peculiarities Stonehouse listed were perhaps better explained as a means to allow rapid growth of the antlers. Clutton-Brock also pointed out that, not only do some deer grow antlers during the winter (Roe deer, for example), when they presumably have little need to lose excessive heat, but also that:
“in tropical cervids there is no close association between antler growth and temperature”
and

“the antlers of temperate deer species tend to be larger, relative to their body size, than those of tropical species”.
An alternative suggestion was that antlers evolved as a method of defence against predators. This was first suggested by Charles Darwin in his 1871 book The Descent of Man and may well be part of the story, although, as with Stonehouse’s theory, this seems unlikely given that only one sex grows them. Indeed, females and their young are arguably more vulnerable to attack from predators during the summer months than stags/bucks are. That said, it is interesting to note that Reindeer females grow antlers during the calving season and, unlike many deer species, the calves accompany their mother as soon as they can walk rather than being left lying in cover while the mother feeds. It seems likely that antlers allow the female to offer an extra element of protection to her calf.
Some authors have suggested that antlers may have evolved as a tool for gaining access to food. Indeed, Reindeer have been seen to dig craters in the snow with their antlers to get at lichens and there are various reports of deer using them to knock fruit, and in the case of one ingenious Red deer stag on Exmoor, bird feeders, from trees. I’ve even seen a report of one deer using its antlers to operate farm equipment to cut some carrots. On reflection, however, it seems unlikely that such tasks would require antlers of increasing size and complexity and a more likely explanation is that the antler can be used to gain access to certain foods, just as they can be used to scratch hard to reach places on the back, but they did not evolve as a response to these tasks.
Tools of love and war
We now arrive at a series of theories that actually tie together under the umbrella of antlers being social apparatus. The most widely regarded explanation for the evolution of antlers is that they may be both an advertisement of fitness and weapons for use in intraspecific combat. In other words, the antlers serve to show how fit and strong a stag/buck is, both to potential mates and other males thinking about launching a challenge, and are used in fights between males for access to resources.
In his 1998 book, Deer of the World, Valerius Geist argued that antlers evolved as a response to feeding out in the open; being out in the open makes you more vulnerable to predators, which permits the formation of herds because more eyes and ears makes it less likely any one individual is going to get attacked. The downside to living in a group is that there are many mouths going for the same food, in other words there’s competition, and this leads to aggression and in-fighting. Geist explained:
“Weapons that maximize wounding ultimately attract predators to the group, disrupt normal functions, and increase the cost of daily living to the group. Statistically, an individual that leaves the group due to wounds or fright reduces the security of each individual remaining in the group.”

So, animals living in a herd need a way of establishing a ‘pecking order’ without causing any serious injury – complex antlers do just this, by allowing largely ‘bloodless’ wrestling and sparring matches. The tips of the antler tines are sharp and capable of inflicting serious injuries, but, as Gerald Lincoln points out in his 1992 review, the junctions between the tines and the main beam of the antlers form pivots to interlock with an opponent’s antlers. In other words, antlers serve to catch the charge of an opponent and hold onto his head so that wrestling can commence. Geist gives a detailed coverage of the evolution of antler forms and the reader is directed there for a more complete picture.
Sufficed to say, the precursors to modern antlers (so-called protoantlers) were bony, skin-covered, hairy extensions of the skull possessed by the mid-Miocene deer Dicroceros elegans and used in defence, their curved upper canines being the offensive weapons. These protoantlers probably evolved into bony lumps on the head as a response to a need for protecting the head against bites. According to Geist, it was the bone protecting the underlying skull structure that was the origin of the early “true antlers”; such antlers are first recorded on small deer from Old World Europe that looked similar to modern day muntjac.

Of course, competition for food is not the only form of contest that herding animals experience; competition for mates is equally important and antlers appear to have evolved to allow males to compete with each other for access to females. Indeed, as Clutton-Block noted in his review:
“The occurrence of antlers in males and their absence in females (who do not have to fight for access to mating partners) is in accordance with the theory that they evolved as weapons.”
The idea that antlers are primarily used as weapons for intraspecific competition can be demonstrated by experimentally removing the antlers of captive deer. Many such experiments were carried out by Gerald Lincoln during the late 1960s and early 70s and the results showed that when antlers were removed, the stags were immediately challenged by other members of the group, suggesting they’d fallen to the bottom of the hierarchy. Similar observations were made by Michael Abbleby working with Red deer in Scotland and by Ludek Bartos and Vaclav Perner on the white Red deer herd kept on the Zehusice Game Reserve in Czechoslovakia.
Red stags form bachelor groups outside of the breeding season and both Abbley and Bartos and Perner reported that as the stags cast their antlers they rapidly dropped in the hierarchy; once all the deer had cast, the pecking order was reinstated. Furthermore, a loss of status seems to translate to poor breeding success in wild populations, because antlerless males cannot compete with antlered ones and thus fail to maintain a harem. Indeed, in a paper to the Journal of Experimental Zoology during 1972, Lincoln wrote:
“... the loss of antlers can radically impair the rutting performance, and prevent animals from taking a harem and participating in mating ...”
The relationship between antler size and fighting ability or dominance is less clear. Some authors, such as Clutton-Brock in his Behaviour review, point out that the relationship between antler size and dominance is not a particularly close one and an individual’s fighting ability changes throughout the rutting season, while his antler size remains constant. There are, however, multiple studies confirming a positive correlation between antler size and body size so if, as the studies suggest, body size is the major factor influencing rutting success, it should be possible to gauge a stag’s fighting prowess based on his antler size.
There is some evidence, from a study using stuffed heads with different antler sizes, that stags can do this, but Clutton-Brock considers that a stag basing a decision whether to fight another based solely on its antlers is likely to make the wrong choice. In their 1982 book, Clutton-Brock and his colleagues point out:
“... among stags over five years old in our study area, neither fighting success nor reproductive success was related to antler length ...”

Indeed, Clutton-Brock questions whether correlations between antler size and dominance, fighting ability and reproductive success are picking up on a relationship that is actually a by-product of the well-known correlation between body size and dominance. This would make sense given that antler breakage doesn’t seem to have any significant impact on rutting performance. In a study of the Tule elk (Cervus cannadensis nannodes), for example, California Fish and Game biologist Heather Johnson and her colleagues found that antler breakage, regardless of severity, had no effect on either the fighting success or harem-holding success of bull elks in Owens Valley, California. Even if breakage were a problem, it appears to be rare and, in a 1971 note to the journal Nature, John Henshaw wrote of how he had only come across one instance of breakage of the main beam in his observations of “approximately a quarter of a million cervids of nine species”.
We still cannot be sure what visual cues females use when assessing a potential mate, or that stags use when assessing a potential challenger, but it is thought that antler size may be part of the story, offering a proxy for fitness. Indeed, in 2005 a team of Spanish biologists lead by Aurelio Malo at the Museo Nacional de Ciencias Naturales in Madrid found that Red stags sporting large, complex antlers had relatively larger testes and faster sperm than those with smaller, simpler appendages.
Subsequently, in a 2007 paper to The American Naturalist, a team of French and Swedish scientists argued that antler size may “provide an honest signal of male phenotypic quality in roe deer” – in other words, Roe does may be able to tell the quality of a buck by the size of his antlers. Thus, females may be able to use the size of a male’s antlers as a cue to their ‘quality’ as a mate, because large antlers generally indicate an animal in good condition.
If females actively choose males with large, complex antlers over those with smaller, simpler ones we should see a selective pressure towards males with large and complex ornaments. Data from the Rum population provide some evidence for this, showing that the mating success of Red stags between the ages of seven and ten years is associated with the number of points on their antlers, those with more points generally having more matings that those with fewer points, although the relationship is not always clear.

Give me strength
Studies on the mechanical properties of antler bone lend additional support to the theory that they evolved primarily as weapons. A study by University of York biologist John Currey and his colleagues assessed how effective antler of varying ‘moistness’ was at standing up to force. The researchers compared various measures of physical strength (elasticity, work-to-fracture ratio, etc.) of antler samples and sections of wet femur; they discovered that antler could withstand just over twice the sustained force (i.e. force attempting to bend the antler to breaking point) and six-and-a-half times more blunt impact force before it broke than wet femur. Similar work by Peter Zioupos at Cranfield University confirms Currey's observations, with Red deer antler found to withstand five-times more sustained force than femur of the same thickness (1.75t vs. 350kg) - high-speed video of the experiment clearly showed the antler bending substantially before finally fracturing. Zioupos also noted an area of porous bone directly below the pedicle that appears to help disperse blunt impact force, dampening the shock to the brain and thereby acting like a "crash helmet" for the deer.
Currey and his coworkers suggested that the antlers begin to dry out once the velvet is shed, but only sufficient moisture is lost to improve the mechanical properties of the bone. Thus, if the antler was too wet it would simply distort under the pressure applied during a clash; but if it were too dry it would too stiff, more brittle and thus more likely to break. Indeed, Richard Prior points to a similar scenario in his 1995 book, suggesting that Roe antlers gain density, perhaps by absorbing resins and so on through fraying. Prior points out that most late-shot bucks seem to have very hard, dense antler compared with those shot just out of velvet, implying a gradual drying out of the antlers following casting.
While working at York University, Zioupos, Currey and PhD student Andy Sedman studied the cracking behaviour of Red deer antler under the microscope – specifically, they looked at “microcracks”, tiny cracks that form in materials but are invisible to the naked eye. The results of their study were published in Medical Engineering & Physics during May 1994 and reveal the crucial role microcracks play in strengthening the antler.
The biologists observed that when a microcrack forms in an antler, unlike in ordinary bone, it takes a “tortuous/devious route”, which dissipates the force across a wider area, allowing it to absorb a greater amount of energy before breaking. Additionally, the microcracks appear more isolated and dispersed in antler than femur bone and the bone canals (osteons) are more prone to detaching their lamellae, further reducing the potential for the microcracks to join-up into a “macrocrack”. In other words, these microcracks “discourage” the antler from fracturing and the more of them there are the more force is dispersed throughout the bone. Indeed, in his thesis, Sedman concluded that the two types of bone fail by two different processes. Ordinary bone sustains damage that causes a single fracture, resulting in it breaking – this is what physicists call a damage related fracture process. Antler bone, by contrast, must sustain lots of small fractures that must join together before a large enough crack forms, a process known as damage coalescence. As Sedman put it:
“… it is antler's ability to accumulate damage far more rapidly than bone can, that is the underlying cause of its greater impact strength.”
Useful by-products
Before we leave the subject of what use antlers may serve, it is worth briefly mentioning work by University of Guelph biologist George Bubenik and Cleveland State University mathematician Peter Bubenik. In a 2008 paper to the European Journal of Wildlife Research these scientists presented data showing that the palmated (broad, flat) antlers of Alaskan moose (Alces alces) might serve as a parabolic reflector of sound. The researchers constructed a ‘fake ear’ and attached it to an antler, pointing it at various different angles. The data show that the loudest response was registered when the ear was facing the centre of the antler; the sound pressure was 119% that of when the ear was facing forwards (i.e. away from the antler) and the authors concluded:
“These findings strongly indicate that the palm of moose antlers may serve as an effective, parabolic reflector which increases the acoustic pressure of the incoming sound.”

In other words, the sound hits the antler and is bounced off into the ear, thereby improving the moose’s hearing. It seems unlikely that antler evolved in moose in response to a need for better hearing, but it reinforces the idea that such structures may convey multiple advantages.
Unexpected antlers
Not all antlers grow and develop in the pattern typical of the species. Sometimes aberrations occur, including a failure to shed one antlers, atypical growth of one or both antlers and antler growth by female deer.
In the Summer 2016 issue of the British Deer Society's Deer journal, a letter and accompanying photos of a roe deer buck shot by Andrew Yool was published. This very curious specimen presented with deformed left antler. The pedicle had grown up and immediately bent at 90-degrees to the skull. Despite this malformation, a normal three-point antler grew, albeit offset some 4.5cm (2 in.) from the root of the pedicle. Of particular interest was that the antler was sill verticle, like a tree growing out of a cliff face bends 90-degrees to grow straight up, raising the interesting question of whether antlers have an internal orientation mechanism.

While issues with sex hormones are typically the reason behind curious looking antlers, sometimes damage to the antler itself during growth can lead to deformity. Indeed, while they’re growing, antlers are very fragile, almost to the point of being a liability to their owner. In a series of rather gruesome anatomical experiments conducted during the late 1970s, Czechoslovakian anatomists Anthony Bubenik and R. Pavlansky found that damage to or breakage of the pedicle can result in severely stunted antlers on that side, while incision in an antler tine resulted in a small scar that grew a small tine the following year. In their paper to the Journal of Experimental Zoology in 1965, Bubenik and Pavlansky noted, however, that antler regeneration couldn’t be halted by trauma in individuals that had passed through at least one antler cycle, although earlier work by Richard Goss has shown that damage to the frontal bone from which the pedicle is derived can permanently inhibit antler growth.
Perhaps one of the most curious aspects of antler biology is when they are grown by animals that don’t generally grow them, or when animals that should normally grow them fail to do so. We have already seen that female Reindeer grow antlers, but they have been documented in other deer too. Most frequently such reports come from Roe deer and, as Richard Prior alludes to in his The Roe Deer book, it is not unusual for older females to possess antlers. Prior explains:
“Old does quite commonly grow short antlers in velvet, usually not more than five centimetres long. They are normal breeders, but probably towards the end of their reproductive life.”
Prior goes on to recount the story of a doe he hand-reared, which developed antlers in velvet from the age of eight; the antlers grew larger in successive years (lengthening to 15cm / 6 in.) and by the time she died the doe had developed an abnormality called ‘perruque head’ (see below). A post mortem of the doe revealed some testicular cells in the vicinity of her ovaries. Indeed, the development of antlers in female deer typically seems to be related to a hormonal imbalance, which appears to occur in old age. Alternatively, it may be that the testicular cells may always be present, but their influence is overpowered by the oestrogens secreted by the ovaries – as the doe ages, perhaps a drop in oestrogen secretion allows testosterone to have an influence.
Either way, in their 2008 review of Roe deer natural history Mark Hewison and Brian Staines note that antlers in does are often associated with hermaphroditism; both ‘true’ (where both male and female genital tissue is present) and ‘pseudo’ (where one sex displays the sexual organs of the other). The biologists also point out that pregnant antlered Roe deer are known and that, although doe antlers generally fail to shed velvet, does in ‘hard horn’ have been reported. Roe are not the only species in which females can uncharacteristically grow antlers and antlers are estimated to occur in roughly 0.1% (i.e. 1 in every 1000) of female White-tailed deer (Odocoileus virginianus).
Antler-less males: the hummel
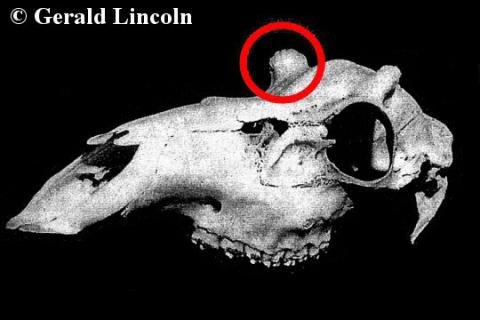
In some cases, males fail to develop antlers and are referred to as hummels or, in parts of south-west England, notts. (It should be noted that true hummels are distinct from haviers, which are antlerless as a result of castration.) Early naturalists proposed that the lack of antlers was either the result of accidental damage to the testicles, or was a heritable genetic condition (i.e. hummels bred to produce hummels). Curiously, all hummel reports I have come across in the literature have been Red deer, although I have heard unconfirmed reports by stalkers that hummel Sika and Fallow deer have been shot in the UK.
Some stalkers considered hummels to have advantage over antlered stags because, spared the energetic drains of antler development, they could grow larger and would therefore be more successful in obtaining a harem during the rut – leading to more hummels. Consequently, hummels tended to be shot on sight in a bid to improve the local trophy standards. In a paper to the Deer journal during 1970, however, Brian Mitchell and Tim Parish presented data showing that hummels do not necessarily grow larger than antlered stags, while the studies by Gerald Lincoln discussed earlier have demonstrated that antlerless stags are at a disadvantage during the rutting season.
Our understanding of hummels took a sizeable step forward when a “congenitally polled” (i.e. antler-less) Red stag was caught at Braemar Lodge in the Scottish Highlands during 1969. Breeding studies conducted by Gerald Lincoln and John Fletcher clearly demonstrated that the hummel condition was not genetic – the stag sired multiple antlered progeny, which also sired antlered stags even when crossed with their sisters (ruling out a recessive gene). This stag also provided scientists with a better understanding of what causes the lack of antlers in hummels.

In 1974, Polish anatomists Zbigniew Jaczewski and Krystyna Krzywinska reported that amputation of the tip of the pedicle of a castrated stag would sometimes cause it to grow an antler, as if the act of wounding the pedicle was the stimulation for antlerogenesis. In a 1976 paper to the Journal of Experimental Zoology, Lincoln and Fletcher presented the results of their surgical study on the Braemar Lodge hummel, which had “rudimentary pedicles” but failed to grow antlers during five years of observation. The biologists found that if they amputated the tip of the stag’s right pedicle, the deer grew a complete, albeit stunted, antler on this side (no growth was documented on the left pedicle) that was subsequently cleaned and cast in the normal way.
The stag died shortly after the experiment and dissection revealed a substantial increase in the thickness of the right pedicle compared to the left. Lincoln and Fletcher concluded that hummels weren’t physiologically incapable of producing antlers; instead a failure to develop fully formed pedicles meant that the antlers had no base from which to differentiate. The researchers wrote:
“... it was possible to induce antler growth in the hummel by apparently simulating the process of ‘wounding’ that naturally occurs at the time of antler casting.”
In other words, it may be the wounding of the pedicle caused when the antler is cast that, in conjunction with a drop in testosterone secretion, triggers the growth of the new antler. The same conclusion was reached by Chunyi Li and James Suttie in their paper to the New Zealand Veterinary Journal in 1996. So, why might stags fail to develop normal pedicles?
Lincoln and Fletcher suggest that, given most hummels are found among the Red deer of the Scottish hillsides, they may come about under conditions of low food availability; low nutrient levels experienced as a calf may starve the pedicles of the minerals needed during the crucial growth period around five months of age. Alternatively, researchers at the Croatian Forestry Society in Zagreb proposed, in a 2008 paper to the Croatian journal Sumarski List, that:
“As pedicle growth depends on androgenic [testosterone] stimulation, low levels of circulating androgens or a low density of androgen receptors in the antlerogenic periosteum could lead to poor pedicle growth and in consequence to a complete or almost complete lack of antler growth.”

Hummels are relatively rare (in 1990, G. Kenneth Whitehead put the figure at less than 1% of Scottish Red deer), so the data we have are from only a few individuals and the results may not be representative of all cases. However, off-hand it seems that, if the hummels were a response to a lack of androgen receptors in the pedicle, this is unlikely to be corrected by simple surgical wounding of the tissue. Ultimately we are still unsure as to the exact cause of this condition, but it seems likely that both early malnutrition and abnormally low testosterone levels are possible contenders. It should be mentioned, incidentally, that although hummels appear more common in Red deer than other species, there are occasional records of Roe bucks without antlers.
Antler over-growth
Antler deformities come in various shapes and sizes and, although none are particularly common, they are typically associated with hormonal imbalances. In her 1991 book, Deer, Norma Chapman notes that the so-called double-head condition is well known in Fallow bucks from Denmark and Germany, with rare reports in German Roe deer and Scottish Red deer. The condition manifests as either a failure to cast the antlers before the new set begin growing, leading to four antlers being present simultaneously, or the formation of an additional one or two pedicles on the skull from which antlers will grow.
The phenomenon of failing to cast before new growth begins can take a more serious turn in a compound-growth condition known as perruque. Perruque, from the French meaning ‘wig’, is a condition where the antlers continue to grow during subsequent years without casting; the result is the growth of what can, in advanced cases, be a rather grotesque and heavy lump of bone that cascades down across the face and obscures the eyes. In particularly warm periods any damage to the velvet tissue, which is often retained indefinitely, may become infected. Perruque seems to be caused by damage to the testicles.

To the best of my knowledge, the only documentation of the onset and progress of perruque in Roe deer comes from this single Roe Prior cared for. Although most commonly reported in Roe bucks, there are occasional records of (less dramatic) perruqueing in Fallow and Red deer.
Roe bucks have also been recorded with coalesced antlers, where the main beams merge into a single thick mass; they are shed as a single unit. Coalescence seems to be the result of larger, thicker pedicles being situated closer together than normal and, in his 1995 book, Prior notes that this condition may be more common in elderly bucks, as the pedicles thicken and shorten with successive antler castings.
Injuries to limbs have also been implicated in the abnormal development of antlers. There are surgical experiments in Sambar deer (Cervus unicolor) and Indian muntjac (Muntiacus muntjak) showing that amputation of part of one hind limb can lead to stunted growth of the opposite antler – amputation of the left hind leg, for example, would produce stunted growth of the right antler. This is often referred to as the contra-lateral effect and, writing in 1966, Anthony Bubenik considered there to be separate ‘antler growth centres’ controlling the growth of each antler independently, although this still has not been empirically confirmed.
In his Encyclopedia of Deer, Whitehead suggests that over-growth on one side may be a form of ‘bilateral compensation’, recounting a case where a Sambar deer grew a left antler three or four times heavier than the right, apparently to compensate (balance out) a weakened, handicapped left hind leg. Similar stories are discussed by John Fletcher in his A Life for Deer, in which he tells how deer stalkers long spoke of how an injured stag would “grow a twisted horn”. Fletcher mentions that, although deer have an extraordinary capacity to repair bone fractures spontaneously, where a fracture coincides with antler growth an asymmetrical antler with distorted growth is almost inevitable; he speculates that this might be related to liberation of endorphins (natural pain killing chemicals) by the body.
Hormones tend to be involved in antler abnormalities in one form or another, but in some cases parasites have been implicated. There are some rare examples of deer (generally Red stags) growing twisted, or corkscrew, antlers; the origin of such abnormalities is currently unknown, but it has been suggested that it may be related to internal parasite load, specifically of lungworms (Dictyocaulus spp.).
Convenient cancers?
In 2016, George Darwall wrote to the British Deer Society in response to a letter published in their Deer journal about why deer cast and regrow their antlers. Mr Darwall pointed out that bovids grow and retain their headgear from birth and, based on distribution and species diversity, appear more successful mammals than deer. The question is, therefore, why it should be necessary for deer to continually shed their antlers, given the huge drain in resource. Horns grow slowly and are of continuous use to their owner, while "an antler is a liability until its rapid growth has stopped and the bone has dried".
Darwall raised the intriguing idea that ancestral deer may have developed an ability to grow tumours from their pedicles; the resulting cancers (antlers) subsequently coming in handy to their owner. Left unchecked, Darwall muses, the tumour could become a serious drain on the deer's resources and prove fatal -- the perruque we see in roe deer, perhaps such an example -- but, as many tumours (e.g. breast and prostate) require a suitable supply of sex hormones to grow, the seasonal breeding cycle of deer may result in the periodic death of the tumour.

I find this suggestion particularly provocative because it actually offers a rare explanation for how antlers evolved, not just why. Invariably there are some outliers here -- muntjac, for example, don’t seem to develop perruque and yet exhibit antler growth that doesn’t appear to be associated with their sexual cycle -- but the science is now starting to flesh out this idea a little further and it turns out that Darwall was on the right lines.
In a fascinating paper to the journal Science in 2019, a team of geneticists, led by Qiang Qiu at the Northwestern Polytechnical University in China, presented data on gene expression profiles and cellular basis for horns in bovids (cows, sheep, goats, antelope, etc.) antler growth in deer. Their analysis identified 624 horn-specific and 761 antler-specific genes that, in both groups, were most highly expressed in the same four tissues: bone, skin, testis and brain/nerve. In other words, genes are “recruited” from these tissues in order to manage the growth of horns and antlers and, in the case of antlers, more are of nervous (143) and testicular (81) origin than for horns (67 and 65, respectively).
A particularly interesting finding in these data was the presence of 201 “headgear-specific” genes that were shared by both antler and horn tissues. This suggests that when both horns and antlers grow, many of the same genes for forming skin, bone and nerves are turned on and the initial process of bone growth is remarkably similar in both types of headgear, implying a common ancestry. This was further supported by their observation that one gene in particular, called RXFP2, that is specifically expressed in both the headgear and testis, was disabled (more accurately, convergently pseudogenized) in the musk (Moschidae) and Chinese water deer (Hydropotus inermis), both of which lack antlers.

Knowing that the growth of horns and antlers is similar and calls on the same genes is fascinating in itself, but what does this have to do with antlers and cancer? Well, Qiu and his colleagues also observed a high expression of genes associated with a couple of very specific gene pathways that regulate cell growth.
The geneticists found evidence that three “proto-oncogenes”, these are genes that can cause cancer by sending cells into overdrive, were under positive selection in early cervids. One in particular, a transcription factor called FOS, has been well studied in humans and mice and is well known to result in tumour growth in its overactive state. As well as lots of FOS activity, a tumour suppressing gene called TP53 was also expressed in antlers. Without getting too bogged down in the detail, TP53 provides the instructions for the manufacture of a protein called p53, which “checks” the genome for damage that might lead to cancer. If p53 finds a damaged segment of DNA, it can call specialised repair molecules, prevent the cell from dividing or, if the damage is beyond repair, trigger the cell’s death. The Chinese scientists found three cofactor and five regulator genes for p53 that were expressed only in antlers.
Taken together, these data do indeed suggest that antler growth is circumscribed osteocarcinoma – essentially, a controlled form of bone cancer. Moreover, the high levels of tumour-suppression genes such as TP53 (and also one called ADAMTS18) may account for the relatively low rate of cancer among deer, with cancer frequency records from Philadelphia and San Diego zoos suggesting cancer rates are five-times lower in deer than other mammals.
Wrapping up
So, in summary we have established that antlers are deciduous bony structures that develop from extensions of the stag’s (and in some species, hind’s) skull. The first antlers are usually simple, unbranched spikes that grow as extensions to the pedicle and thus lack a coronet; subsequent antlers are progressively larger and more branched. Casting and re-growing of the antlers is under seasonal influence, which acts upon androgen levels in the deer’s blood – in spring, low testosterone levels cause the antlers to be cast and new growth to start, while in the autumn a rise in testosterone (in preparation for the rut) causes the velvet to be shed.
There are times when antler development goes awry and these are generally associated with hormonal imbalances, as are the atypical growth of antlers in females. Antlers are used as weapons during combat between stags for mating rights and, in reindeer, may help excavate food buried under the snow and compete with bulls for access to precious winter resources.
Antler Growth References
Bauer, E.A. (1995). Antlers: The antlered animals of Europe and North America. SwanHill Press, Shrewsbury.
Chapman, D.I. (1975). Antlers - bones of contention. Mamm. Rev. 5(4): 121-172.
Goss, R.J. (1983). Deer antlers: Regeneration, function, and evolution. Academic Press, USA.
Goss, R.J. et al. (1992). The mechanism of antler casting in the fallow deer. J. Exp. Zool. 264(4): 429-436.
Kierdorf, U. et al. (2009). Improbable appendages: Deer antler renewal as a unique case of mammalian regeneration. Sem. Cell Devel. Biol. 20(5): 535-542.
Li, C. et al. (2007). Antler Regeneration: A dependent process of stem tissue primed via interaction with its enveloping skin. J. Exp. Zool. 307A(2): 95-105.
Price, J.S. et al. (2005). Deer antlers: a zoological curiosity or the key to understanding organ regeneration in mammals? J. Anat. 207: 603-618.
Putman, R. (1988). The Natural History of Deer. Comstock Publishing Associates, New York. (Chapter 7).