Elasmobranch Senses
Elasmobranchs have an awe-inspiring battery of senses that help them locate and catch their food.
Vision
In his 1974 bestseller Jaws, the late Peter Benchley wrote of the white shark: “The eyes were sightless in the black and the other senses transmitted nothing extraordinary to the small, primitive brain.” Contrary to this popular misconception, which remained dogma for many years, sharks have good eyesight and many even seem to have limited colour vision.

The structure of an elasmobranch eye is similar to that of most vertebrates, although there are some differences. Probably the most obvious difference is that elasmobranch lenses are larger and almost spherical in shape while most vertebrates have a smaller, stretched lens that appears oval. The seawater and the shark cornea, the transparent membrane in front of the eye, are roughly the same density (unlike land-based vertebrates, which have a cornea more dense that the surrounding air) meaning that the cornea cannot contribute to the focussing of an image, leaving that entirely up to the lens. Consequently, a large powerful lens is required, although rather than changing the shape of the lens as humans do, elasmobranchs change its position – the lens moves back to focus on distant objects and vice versa.
Studies conducted by Samuel Gruber and funded by the Office of Naval Research in the US during the mid- to late 1970s were instrumental in dispelling the myth that sharks have poor eyesight. One specific paper of Gruber’s, published in 1985, looked at the visual system of the great white shark in detail. Gruber and his co-workers found that the white shark had the lowest rod (used in low light conditions) to cone (involved in detection of colours in bright light) cell ratio of any shark studied to that point. The great white apparently has a rod:cone ratio of 4:1 (i.e. for every four rod cells, there is a cone cell); most sharks studied to that point had ratios around 10:1. The upshot of this is that the great white seems to have the best bright light (probably also colour) vision of the 15 elasmobranch species for which Gruber et al. had data. Sharks are also able to see distances of up to 20m (almost 66ft) depending on water conditions.
As one might expect for a crepuscular predator, most shark eyes are adapted for low-light conditions. Lying immediately behind the retina is a layer of plates, covered in a guanine-like crystalline substance, called a tapetum lucidum (a Latin phrase meaning “bright carpet”) that acts as a kind of mirror to reflect light that would otherwise pass through the retina (and be lost) back into the eye. This a very similar mechanism to the one that causes the familiar “eye shine” in most other mammals. In bright conditions, special cells called melanoblasts slide from the base of each of the plates, covering it completely and preventing the shark from being dazzled. During dark or poorly illuminated conditions, the melanoblasts are drawn back, exposing the silvery plates. Therefore, elasmobranchs are able to utilize more of the available light, giving it a significant advantage during night-time hunts.
Most of the studies looking at vision in elasmobranchs have used restrained sharks and these fish have always been shown to be slightly hyperopic (longsighted); thus, they were thought to have difficulty focusing on close objects. More recent studies by Robert Hueter at the University of Florida have, however, shown that restraining sharks may cause them to contract their lenses, giving a false impression of farsightedness. By bouncing beams of infrared light off the retina of free-swimming juvenile lemon sharks, Hueter and his colleagues were able to demonstrate that the sharks were able to focus on both near and distant objects.
Smell (Olfaction)
Sharks are notorious for their sense of smell, and seldom is a discussion on sharks complete without some mention of how “a shark can smell a single drop of blood in an Olympic-sized swimming pool”. An Olympic swimming pool contains about one megalitre (one million litres, or almost 265, 000 gallons) of water. Side-stepping the indefinite volume of “a drop” and assuming a large drop of 1ml, sharks should thus be able to detect a single drop of blood in 1,000 million drops of water. More commonly, the figure presented is closer to a single drop in about 90 litres (25 gallons) of water – i.e. one molecule of blood in a million molecules of water.
There are few laboratory studies that have looked at just how accurate such statements are, and most have been carried out on batoids. On his ReefQuest site, Aidan Martin noted that certain rajoids (skates) can detect concentrations as low as one molecule of the amino acid serine in 1,015 molecules of water. Studies on the morphology of elasmobranchs by Vera Schluessel and her colleagues suggest that olfactory sensitivity is related to habitat more than size or taxonomy. One study, published in the Journal of Morphology during 2008, concluded that benthopelagic species (i.e. those active throughout the water column, from the surface to the sea floor) had significantly larger olfactory surface areas than those living and feeding only on the sea floor. A subsequent study by Trish Meredith and Stephen Kajiura found a similar trend, with bottom-dwelling species having fewer olfactory lamellae than the benthopelagic species they studied.
It was originally thought that as much as 70% of a shark’s brain was put aside for olfactory purposes. A more recent study has shown, however, that this is much lower and highly variable. The largest proportion devoted to smell was 18%, observed in Carcharodon carcharias by Leo Demski and Glenn Northcutt in their 1996 paper on the brain and cranial nerves of the white shark. A whaler shark (Carcharhinus) was found to have 3% of its brain devoted to smell processing, a dogfish (Squalus) 6% and catsharks (Scyliorhinus) 14%.
Shark nostrils, called nares, are blind sacs; they don’t connect to the respiratory tract and therefore have no function in breathing, as they do in mammals. The entrance to each nare is partially obscured by a flap of skin called “Flaps of Schneider” that directs the water through the nare in a sigmoidal (s-shaped) motion. The nare is lined with convoluted lamellae (plates of tissue) onto which free amino acids attach, triggering an electrical impulse to the shark’s brain.

Historically, the bizarre laterally-expanded prebranchial cephalofoil (i.e. hammer-shaped head) of the hammerhead sharks (Sphyrinidae family) was thought to have evolved to provide these sharks with an increased area over which to detect smells – the so-called “enhanced olfaction” hypothesis. Recent research by scientists at the University of California has suggested, however, this is unlikely to be the reason. The researchers found that none of the eight extant (living) species of hammerhead shark were better at detecting scent that their ‘smaller headed’ carcharhinid relatives, and that the sphyrinids and carcharhinids had the same sized surface area of olfactory epithelium, despite the hammerheads having more structural lamellae.
Taste
A more detailed variant of smell, hence if you get a cold and lose your sense of smell you also tend to lose your taste sense, sharks have taste buds located just behind their teeth. In a similar manner to that observed in humans, dissolved chemicals attach to the epithelium of the taste bud and generate an electrical impulse to the brain.
Touch
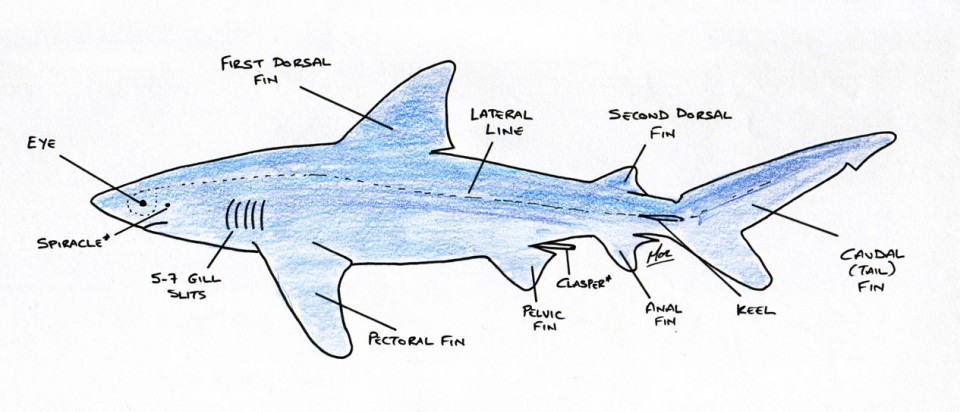
All fish have two types of touch sense: contact tactility and distant tactility. Contact tactility, as the name suggests, is where nerves in the skin fire upon the application of pressure – i.e. to the fish physically touching, or being touched by, an object. Distant tactility is achieved via a lateral line system that’s sensitive to changes in water pressure. The lateral line is a row of pits (pores) that encircle the fish’s head and continue in a single line along either side of the body to the tail. Inside each pore is a sensory neuromast that detects changes in water pressure through the distortion of a hair. If the fish approaches an object, the water between the two objects gets forced into an ever-decreasing space, increasing the water pressure; the fish can detect this and judge the object’s proximity. The lateral line can be used to detect vibrations from objects up to 200m (656 ft.) away.
There is also some evidence to suggest that the lateral line pit neuromasts may be involved in the taste process. Back in 1967, Yasuji Katsuk and a team of scientists from the University of Hawaii were able to demonstrate that shark pit organs responded not only to changes in ambient salinity, but also showed a dramatic neural response to meat and blood. The teeth of sharks are also highly innervated, and some consider that, without the benefit of hands, they use their extremely dextrous jaws to investigate unfamiliar objects in their environment.
Electroreception
Elasmobranchs possess the rather special ability -- shared by only a few other creatures, such as the duck-billed platypus (Ornithorhynchus anatinus) -- of being able to detect electric fields generated by other living organisms. The precise mechanisms and details of this get complicated, so I will simplify it for the purpose of this summary.
The sense works via a series of pores, called the Ampullae of Lorenzini (named after Stefano Lorenzini who first described them in 1678), distributed over the shark’s head. According to a paper presented to the 2003 AES Meeting in Brazil by Darryl Whitehead of the University of Queensland, these pits can average as many as 2,052 in the bull shark (Carcharhinus leucas). Each pore leads to a canal lined with a potassium-rich jelly and into a sac containing a receptive hair cell. These cells are receptive to weak DC and low frequency (1 to 4 Hz with rapid high-frequency drop of at 16 to 20 Hz) AC fields.
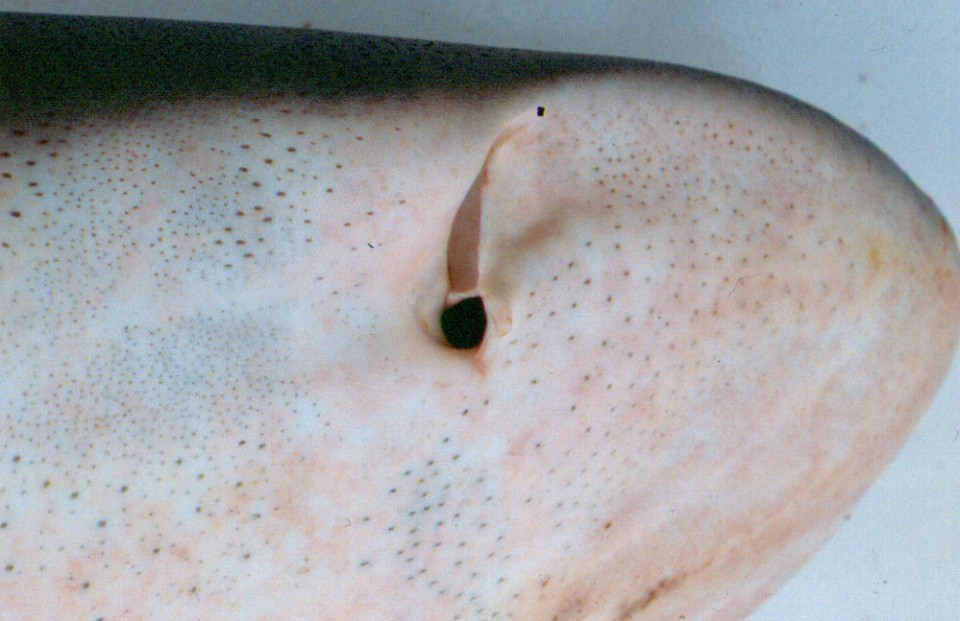
Various studies by Adrianus Kalmijn at the Scripps Institution of Oceanography in California have shown that the elasmobranch electrosense is very sensitive, with some able to detect electrical activity down to five billionths of a volt at a distance of up to 33cm (1ft). To put this into perspective the movement of a plaice’s gill cover as it breathes generates an electrical signal 5 million times higher than this minimum threshold. Electrical charges tend to dissipate readily in seawater, however, meaning that the ampullae are only accurate to a distance of 20 to 30cm (8 to 12 inches) from the object.
As well as detection of potential food, it is also believed that sharks can use their ampullae to detect the Earth’s magnetic field, which they may use to navigate an otherwise feature-poor ocean, and may also be used in mate recognition. An intriguing paper, published in Nature, suggests that sharks are also able to detect changes in temperature with their ampullae.