This is an incredibly complex subject and research is underway all the time, making it virtually impossible to maintain a constantly up to date and accurate resource on this topic without devoting pretty much all my time to reading epidemiology journals. Differing interpretations of data by scientists and politicians have served to muddy the waters, too, making it more difficult than it perhaps should be to establish the facts. Nonetheless, that which follows is aimed at answering some of the questions I frequently see asked on Internet forums. I have tried to maintain the same style that I have used for the rest of the site, without basically turning it into a literature review and bibliography. In some cases, however, I have made more general comments without citing a specific source. As always, I’m happy to provide references for this information to anyone interested in reading the original research.
For a more comprehensive appraisal of the topic and the anti-cull argument I recommend reading Martin Hancox’s article to The Ecologist in May 2016 (Alas poor Brock!). Similarly, Tom Langton provides an anti-cull appraisal off the back of the recent Bovine TB Conference in London in an article to The Ecologist (Bovine TB summit: science-based policy, or policy-based science?) published during April 2017. The broadly pro-cull argument can be reviewed on the TB Free England site – it’s not obvious, but this site is managed by the National Farmers’ Union. The UK government has its own website on bovine tuberculosis containing the official policy and associated statistics. Finally, Andy Robertson's website, TB Knowledge Exchange, was recently brought to my attention. Dr Robertson is a badger ecologist at the University of Exeter and his site provides an easily-accessible and authoritative overview of the mind-boggling statistics as well as a series of short fact sheets explaining aspects of TB survival, transmission and testing as well as culling and vaccination.
A good literature review was published in March 2011 by biologists at the Agri-food and Biosciences Institute in Northern Ireland – much has happened since, but it provides a thorough overview of the history of the disease and attempts to study and fight it and contains a comprehensive bibliography of the primary literature. A more recent review was published in the journal Epidemiology and Infection in 2016 by Jennifer Broughan and colleagues – the published paper is behind a paywall, but the accepted proof is available on Jennifer’s ResearchGate profile.
Table of contents
- What is tuberculosis?
- What is Bovine TB (bTB) and why is it a problem?
- How do we test cattle for TB and what is a ‘herd breakdown’?
- If farmers are compensated for their lost livestock, why is it such a big deal?
- Where do badgers fit in?
- Are badgers the only potential transmitter of bTB?
- Why don’t we just vaccinate cows against TB?
- Can’t we vaccinate badgers, instead?
- Are DEFRA culling badgers at the moment?
- Culling badgers in Ireland has reduced their incidences of TB in cattle, right?
- What’s the big deal with culling badgers?
- Hasn’t the badger population exploded in the last decade? Surely, without any predators, their numbers need controlling?
- So what is the solution?
- External Links
What is tuberculosis?
Short Answer: Tuberculosis (or TB for short) is a bacterial infection of the respiratory system. The bacteria cause damage to the lungs and infection may spread elsewhere in the body causing multiple organ failure. Symptoms include lethargy, swollen lymph nodes and a hacking cough.
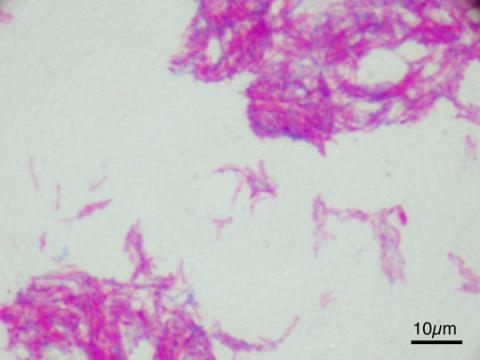
The Details: Tuberculosis is an infection of the respiratory system caused by bacteria of the genus Mycobacterium. When the bacteria enter the host’s lungs they reproduce and cause severe pulmonary distress, creating lesions in the lung tissue; they may subsequently spread to the lymph nodes and to other organs in the body. The disease can cause lethargy, loss of appetite, swollen lymph nodes (particularly in the neck) and a persistent hacking phlegm cough; but the disease progresses slowly which means symptoms often only manifest in the final stages of infection. In humans, a course of antibiotics is typically sufficient to remove the infection.
In humans, TB is typically caused by M. tuberculosis, but there are several closely related bacteria that can elicit the disease in other mammals. M. bovis is typically an infectious agent of cattle, but has the potential to cause TB in humans as well. During the 1930s, the incidence of M. bovis in children (and cats) was high and the bacterium was contracted through the consumption of unpasteurised milk. The implementation of milk sterilization and pasteurising led to a dramatic decline in human bovine tuberculosis (bTB) cases, although people in prolonged contact with cattle or their meat (e.g. abattoir workers) have been known to contract M. bovis infections. According to the Institute of Animal Health in Berkshire, globally around 2,000 people currently die of bTB each year. TB, as a complex, is the world’s most significant bacterial pathogen, estimated to kill three million people annually.
What is Bovine TB (bTB) and why is it a problem?
Short Answer: bTB is a form of tuberculosis caused by the Mycobacterium bovis bacterium. It infects cattle and can spread within a herd and between neighbouring herds quickly, resulting in many animals having to be slaughtered. This is both traumatic and expensive. Indeed, bTB outbreaks are estimated to cost the UK tax payer at least £1 billion over the next decade.
The Details: Bovine tuberculosis is a tuberculosis infection caused by the bacterium Mycobacterium bovis. The disease causes similar symptoms to those described above and, although treatment in cattle is potentially possible using the same suite of antibiotics that are prescribed to human sufferers, treatment is generally considered too costly and takes too long (allowing the bacteria to spread) to be economically viable and infected animals are culled from the herd. Furthermore, treatment options may be more limited in cattle as there is some evidence that M. bovis has developed a resistance to at least one of the antibiotics (pyrazinamide). There are data suggesting that certain breeds may be more susceptible to the disease than others (zebu cattle in Africa and Holstein Friesians in India, for example, appear significantly more resistant to bTB than European breeds such as Jersey cattle) and disease susceptibility seems to increase with age and possibly dominance rank within the herd. To the latter point, a study in New Zealand found almost 90% of the cattle testing positive for bTB were the most dominant fifth of the herd and it was dominant animals that were most likely to most actively investigate the sedated possum (a wildlife carrier in NZ) put in their enclosure.

The bovine TB statistics are staggering. Currently, the UK experiences between about 4,500 and 5,000 new herd incidents per year, most in England. In 2016, for example, there were 4,499 new herd incidents, of which 3,745 (83%) were in England, 710 (16%) in Wales and 37 (1%) in Scotland, a split that is also representative of the past decade. England also has the greatest proportion of registered cattle herds, standing at 51,000 in 2016, compared with 11,600 in Wales and 13,300 in Scotland. Between 2005 and 2016, some 915 thousand British herds, amounting to almost 89 million cattle, have been tested for bTB infection. The result has been almost 55,000 herds having been identified has having the bacteria and nearly 405 thousand cattle being sent for compulsory slaughter. In 2016 alone 39,390 cattle were killed having tested positive for bTB, which is just over 2% of the 1.8 million cattle that Defra estimated constituted the UK’s dairy herd in the same year.
Culling 2% of the UK’s dairy herd (or 0.4% of the UK’s total cattle population, which Defra estimated to be 9.8 million in 2016) may sound relatively trivial, but the outbreaks are clustered making some local percentages much higher. In Cornwall, for example, 95% of the 1,000 dairy herds with 50 or more cows had some form of movement restriction and/or compulsory slaughter imposed on them as a result of cattle testing positive for TB between 1990 and 2012. Indeed, large areas of south-west England, central western England and Wales are currently badly affected. Overall, herd breakdowns, cattle dispatching, compensating farmers, surveillance, etc. is estimated to cost the UK almost £90 million per year. A recent report by the House of Commons Environment, Food and Rural Affairs Committee estimated that bTB has cost the UK taxpayer more than £500 million in the last decade and predicts that it will cost more than £1 billion over the next 10 years.
Instances of TB infection in cattle rose from the mid-1980s, with significant peaks during 2001, 2005 and 2008 associated with the Foot and Mouth outbreak, which suspended cattle controls and appeared to result in a widespread increase in bTB prevalence in both cattle and badgers, and changes to way parishes were tested. There has been an overall long-term upward trend in the incidence of bTB in English and Welsh herds since the mid-1990s, when Defra’s statistical series began, although there is some evidence that the rate of new incidents is levelling off in most areas.
How do we test cattle for TB and what is a ‘herd breakdown’?
Short Answer: Most cows are tested using the SICCT test, also known as the skin tuberculin test, which involves injecting Mycobacterium extracts called tuberculins into the cow’s neck and measuring the response after a few hours. The size of the swelling at the injection site determines if the cow is a “reactor” and any single reactor results in suspension of the herd’s TB-free status – this is known as a herd breakdown.
The Details: There are two main tests currently used to diagnose TB in cattle. Most cows are subjected to the Single Intradermal Comparative Cervical Tuberculin test (SICCT or skin tuberculin test, for short), which involves injecting Mycobacterium protein extracts called tuberculins into the cow’s neck. The first injection contains M. bovis tuberculin and the second, administered at the same time but about 13cm (5 in.) above the site of the first, contains M. avium tuberculin. Cows infected with bTB already have immunoglobulins circulating in their blood and will quickly launch an immune response to the tuberculin, causing the injection sites to swell up. The difference in the amount of swelling at the two injection sites is measured and compared 72 hours later and indicates whether the animal is infected with bTB or some other strain.
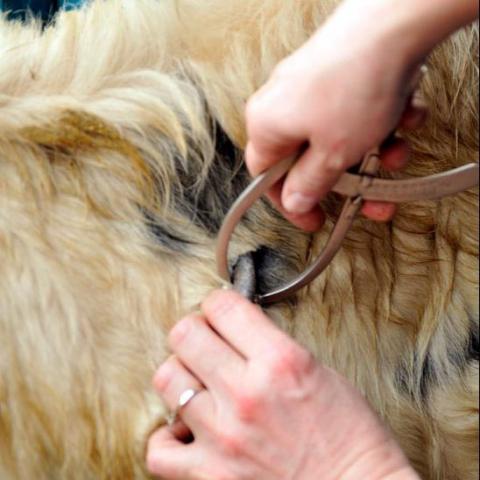
A cow is classified as positive for bTB (a reactor) if the bovis swelling is more than 4mm larger than the avium swelling, negative if there is no reaction, or inconclusive if the difference between the bovis and avium swellings is 4mm or less. Reactors are removed from the herd and valued, in order to pay compensation, before being sent for compulsory slaughter. Inconclusive animals are usually re-tested after 60 days. If a reactor is found within a herd, movement restrictions are placed on the farm and it loses its “TB-free status”, which means no cattle can be brought on to or taken off the farm until it has been declared TB-free. When one animal is confirmed to have TB and the TB-free status is withdrawn it is known as a herd breakdown.
With any diagnostic test, there is a trade-off between sensitivity and specificity and critics of the SICCT test point out that although it’s 99.98% specific, it is only 65% to 80% sensitive. The test’s sensitivity tells us how likely it is to correctly diagnose an animal as infected (i.e. how accurately it picks out the infected animals from uninfected ones); the higher the percentage the less likely it is to get the diagnosis wrong. A test’s specificity tells us how good a test is at correctly identifying animals that are clear of the disease as being healthy; the higher the percentage, the less likely it is to tell us an animal has the disease when it doesn’t. The difference between these two measures is subtle but important because, from a disease control perspective sensitivity is more important than specificity.
Let’s say we have a pretty non-specific test, only 50% specific; if we test a herd of 1,000 cattle all of which are actually free from bTB, up to 500 could show up as having the disease even though they don’t. The result is a herd breakdown during which 500 cattle are killed unnecessarily and movement restrictions are placed on the farm. This is devastating for the farmer, but holds no epidemiological concern because there was no disease to spread in the first place. The bigger issue is when a test has a low sensitivity, because it’s potentially giving infected animals a clean bill of health, leaving them in the herd to spread the disease further. So, in the case of SICCT, the chance of a herd being subject to an unnecessary breakdown is low (0.02% or 1 in 5,000) because the test is 99.98% specific. The sensitivity of 65-85% is, however, more of a problem because it means that 15-35% of the herd may have bTB but may not be picked up by the test; they thus don’t get removed from the herd and can infect other cows.

Factors such as pregnancy, which suppresses the cow’s immune system, certain parasites (particularly infection with the liver fluke, Fasciola hepatica) and even the production of the test can all affect the SICCT test’s effectiveness. Indeed, tuberculin manufacture has been described as much an art as a science. Consequently, in some cases, the interferon gamma (abbreviated to IFN-γ) blood test is also employed; it’s widely considered more sensitive (80-90%) than the SICCT test and can detect infection at an earlier stage. IFN-γ tests do, however, need to be conducted in a laboratory because they involve incubating a blood sample to culture the bacteria, adding to the time and cost. In August 2016 Defra launched a consultation with a view to using the IFN-γ test more widely. Recent work by the Royal Veterinary College’s Svetlana Buzdugan and colleagues suggests that both tests should be used, the results analysed in parallel, when testing for TB in badgers and this may also apply to testing cattle.
More recently, in a paper to the journal Virulence published during May 2016, a team led by Benjamin Swift at Nottingham University presented the results of initial experiments using a bacteriophage PCR test to detect M. bovis in cattle blood samples. Early results suggest this test may offer a sensitive and rapid (results within 48 hours) method for detecting the bacteria in blood sample without the need for culturing.
If farmers are compensated for their lost livestock, why is it such a big deal?
Because most farmers aren’t the heartless monsters that they are often portrayed to be. Having spoken to several on this subject they have impressed two points on me: that the financial compensation is based on the most recent table of market valuations made by Defra, and may not represent what they would actually get for the cow at market; and the considerable emotional cost associated with losing the livestock, which can be devastating, particularly during serious or repeated outbreaks. Furthermore, the imposition of movement restrictions means that the farmer cannot sell any cows, nor add to his stock, which can impose further financial costs and add to the stress. Taking a broader view; the compensation, along with the cost of testing and cattle destruction, is a considerable financial burden on the UK tax payer.
Where do badgers fit in?
Short Answer: Nobody is 100% sure, but badgers are known to be carriers of the disease – unlike most mammals, which succumb quickly to infection, badgers can live with the disease for many years during which some may also shed the virus in their faeces, urine and breath. Cattle encountering infected badgers or pasture may then contract the disease. It’s a very complicated and controversial picture and there’s much we don’t know, but it is suggested that badgers may sustain a wildlife reservoir of the disease, allowing it to pass back into cattle herds sharing the same pastures. The NFU, for example, maintain that TB in cattle cannot be eradicated without also removing it from the wildlife reservoir (by culling badgers).

The Details: This is the million dollar question. Badgers, like all mammals, are susceptible to infection by M. bovis and seem to lack an innate resistance to the bacteria. Indeed, recent work by a team of Oxford University biologists, led by Kirstin Bilham and published in Scientific Reports in April 2017, suggests badger immune cells, called macrophages, don’t react as we’d expect them to when confronted with M. bovis and that this impairs their capacity to resist bTB infection. Unlike most mammals, however, the disease tends to progress very slowly in badgers, such that they may live with infection for many years without any obvious clinical signs. The concern is that, during this time, they can spread the infection to other badgers and any cows in their territory.
The first record of tuberculosis in a European badger (Meles meles) came from the Basle region of Switzerland in 1957 and there was no indication that this was connected with bTB in cattle; roe deer and chamois had previously been found suffering from bTB in the same area and were thought to have been the source of the badger’s infection. In Britain, a comprehensive programme of cattle testing and culling had been making significant progress in reducing bTB in the country since the early 1960s, but infections persisted in south-west England where several ‘closed herds’ continued to experience breakdowns, fuelling speculation that something else was sustaining the disease. The first indication that it might be badgers came in April 1971, when a badger found dead on a farm in the Cotswold Hills in Gloucestershire tested positive for the disease. Indeed, in their paper to Veterinary Record in 1974, Roger Muirhead and his colleagues presented their findings from post mortem of this and other badger carcasses collected from the county between 1971 and 1973. Tuberculosis lesions were found in 36 (22%) of the 165 badgers they autopsied and M. bovis bacteria were isolated from 12 (11%) of 112 faecal samples they collected. The tuberculous badgers were recovered from 23 separate locations in Gloucestershire, 17 of which were areas with persistent bovine TB infections.

In their paper, Muirhead and his co-workers suggest that badgers may be playing a role in sustaining the infection by acting as a wildlife reservoir for the bacteria. Subsequently, by the early 1980s bTB infection had been documented in many other counties in south-west Britain and parts of Ireland and, in a report for the Ministry of Agriculture in August 1980, then president of the Zoological Society of London Lord Solly Zuckerman concluded that badgers were a significant reservoir of bTB in south-west England.
Initially, we thought the picture of infection was relatively straightforward. Infected cattle excreted the Mycobacterium in urine, faeces and mucus on to feed and pasture, from where it was picked up by badgers. Infected badgers would subsequently excrete the bacteria in a similar manner, leaving the bacteria in pasture they shared with cows and when visiting farm buildings and water troughs during their nightly wanderings. This would allow the disease to spread to other herds in the area and by moving between the local cattle and badgers the disease could not only persist (with badgers re-infecting herds after their recovery from a breakdown) but also to move further afield as badgers passed it to neighbouring clans at border latrines or while fighting. Based on this idea, it was suggested that it would be virtually impossible to eliminate bTB in cattle all the time there was still a wildlife (badger) reservoir to keep transmitting it back to the cows. As such it was seen as necessary to tackle bTB in both wildlife and livestock.
The real problem with the foregoing is that it’s largely speculation and, even today, we still don’t know very much about how bTB moves about within and between cattle and wildlife reservoirs. Indeed, Professor Glyn Hewinson, the Chief Scientist at the Animal Health and Veterinary Laboratories Agency, summed the situation up quite nicely recently when he told the House of Commons Environment, Food and Rural Affairs Committee:
“One of the real evidence gaps is how much TB is given from cattle to badgers, how much TB is given from badgers to badgers, how much TB is given from badgers to cattle and how much TB is given from cattle to cattle.”
Experiments led by Tony Little at the Central Science Laboratory (CSL) in York in the early 1980s demonstrated that infected badgers could transmit bTB to cows, with five of seven calves showing signs of exposure or infection after up to six months of having been housed with diseased badgers. The data from this study, published in the Veterinary Record in 1982, suggest that badger-to-cattle transmission of TB is possible (probably indirectly via water or hay contamination), but not particularly efficient.
So far as I know, this was the first empirical evidence implicating a direct infection pathway between badgers and cattle, but even 35 years later we still don’t know how likely this transfer is to happen in the wild, if it even does. Proponents of the idea point to genetic studies in both Britain and Ireland that show M. bovis strains isolated from badgers and cattle in the same area are often very similar as evidence that the disease moves between the species; but these studies don’t tell us the direction of infection. Nonetheless, two routes of infection are generally suggested: indirect contact via infected food, water, pasture, etc.; and direct contact between infected badgers and cattle.
We know that infected badgers can excrete a substantial number of bacilli in their urine, faeces and mucus, while infected animals may have a significant bacterial load in their mouths that may be transferred, via bite wounds, from badger to badger. Indeed, the finding of significant numbers of badgers with tuberculous bite wounds was originally reported by former CSL biologist John Gallagher and colleagues in 1976. These lesions were typically superficial abscesses, associated with two puncture wounds approximately three centimetres (one inch) apart. Gallagher and his coworkers suggested that badgers with severe pulmonary disease may have highly contaminated mouths and subcutaneous or intramuscular inoculation of infection arising from bites by such animals is likely to result in more rapid generalisation and extensive disease. In addition, during his Ph.D. thesis at Bristol University, Julian Brown observed that badgers dribble urine while walking, producing urine trails and potentially resulting in the contamination of large areas. The situation is not as straightforward as initially suspected, however, and research on a naturally infected badger population at Woodchester Park in Gloucestershire has shown that different individuals can have different ‘states’ of infection, affecting whether or not they’re a potential transmitter of the bacteria, with sex and age also playing a part.

Research by biologists at the CSL, led by David Wilkinson, has shown that female badgers seem to cope better with bTB infection than males, for example. The same dataset has also identified badgers within the population that were infected with but were not excreting M. bovis living alongside those that were infected with and excreting the bacteria. In other words, infected badgers were not necessarily infectious badgers. The researchers also found that badgers excreting bacilli had a significantly higher mortality (i.e. died younger) than either those that were infected but weren’t excreting the bacilli or uninfected individuals, the latter two groups exhibiting roughly the same mortality, suggesting that the infectious state is life-limiting in badgers.
Despite this difference in mortality, based on a computer model, Jenni Macdonald at the University of Exeter and colleagues suggested that badgers have very stable population dynamics and, because TB doesn’t affect sow fertility, individuals lost to slowly progressing TB can be easily replaced by cubs born to the clan. These cubs subsequently become infected and the disease persists within the group. While TB infection may not affect female breeding, there are data from Ireland linking irregular baculum development and incidences of bTB infection, suggesting that infection may affect male reproduction. The susceptibility to M. bovis infection may also be related to age, although studies to date have yielded mixed results. One 1991 paper reported that infection rate was slightly higher in cubs than in adults (46% and 39%, respectively), while another paper from 1998 described the opposite (13% of cubs and 20% of adults).
How long excreted bacilli survive in the environment and how likely they are to reinfect cattle or other badgers is also largely unknown. Early work by Central Veterinary Laboratory bacteriologist Clifford Wray in the 1970s suggested that M. bovis bacilli were very hardy and could survive for long periods outside the host, but this is based on experiments carried out under laboratory conditions. A team led by University of Warwick biologist Jamie Young found that the bacteria could survive in cool, moist soil for up to 21 months. Nonetheless, studies looking at survival of the bacteria on grass contaminated with badger urine suggest a survival of less than three days and Martin Hancox told the House of Commons Bovine TB Session 2013-2014:
“The widely held belief that cows catch TB from badger urine with 300,000 bacilli/cc is widely improbable. Some 99% drains straight into the soil; the rest is disinfected by UV in sunlight within 3 days, so a cow is unlikely to ingest the minimum dose c1 million bacilli, ie 3cc of fresh urine.”
The bacteria may persist for longer in faeces, although this is also likely to vary considerably with environmental conditions. In their 2000 paper to Research in Veterinary Science, Central Veterinary Laboratory biologists John Gallagher and Richard Clifton-Hadley note that almost 90% of bacilli in badger faeces had died two weeks after being deposited, and 99.7% are gone by four weeks. Hayley King, at the University of Warwick, and colleagues found that significantly more bacterial DNA was present in badger faeces collected from Woodchester park during the summer than in any other season, suggesting that contamination risk may fluctuate throughout the year. The spreading of silage, in which M. bovis can survive to up to six months, has also been associated with an increased risk of infection.
Tony Little and colleagues, in their 1982 Veterinary Record paper, suggested that contamination of water troughs by badgers may be more of an issue than contamination of pasture, because the drinking action of cows could produce an aerosol that would allow the bacilli to enter their lungs. Indeed, in their 2011 review, Adrian Allen and colleagues at the Agri-food and Biosciences Institute wrote that it was difficult to envisage how the necessary aerosolisation of bacilli from these indirect sources (infected soil, pasture, food, etc.) happens in order to infect cows. We do know that badgers will visit and use water troughs and, during his studies in Woodchester Park, Ben Garnett found that troughs needed to be at least 115cm (3ft 9 in.) off the ground in order to prevent badgers from climbing up to them; this also puts them out of the reach of calves, though.
Observations on wild badgers in Gloucestershire and on the University of Reading’s farm during the late 1980s by Paul Benham and Donald Broom suggested that cattle generally avoided grass soiled by badger faeces and urine; the presence of faeces stopped 99% of their 240 cattle subjects eating the grass for almost a month, while almost 90% avoided grass contaminated with badger urine for two weeks. Not all were put off, however, with two willing to graze close to pasture contaminated with faeces and seven grazing close to the urine-contaminated grass. This further supports the idea that transmission from badgers to cattle is possible, but either unlikely or inefficient.

Interestingly, subsequent work by Michael Hutchings and Steve Harris, published in the Veterinary Journal in 1997, suggests that overgrazing is likely to force cows to eat contaminated pasture and low-ranked members of a herd are more likely to graze on contaminated pasture than more dominant animals. Remote camera studies have demonstrated that badgers sometimes visit farmyards in Britain and may potentially contaminate cattle feed in the process, although how significant such contamination is in the transmission of bTB has yet to be established.
Finally, the potential for direct contact between badgers and cattle, where the two species get within the 1.5m (5 ft) or so that we think is necessary to transfer the M. bovis bacteria via their breath, is unclear. A study led by Zoological Society of London biologist Monika Böhm, and published in the journal PLoS One in 2009, used data-loggers to monitor contacts between badgers and cattle in the North York Moors National Park, northeast England. The researchers found that five of the 15 collared cows came into close contact (between 1.5 and 2.5m) of collared badgers during their six month study period. They also observed that some badgers were more likely to interact with cows than with other members of their clan, and that cattle higher in the herd hierarchy were more likely to have contact with both badgers and other members of the herd, offering the most significant potential for bacterial transmission. More recently, however, work led by fellow ZSL biologist Rosie Woodroffe, published in the journal Ecology Letters in 2016, suggests that, although badgers and cattle use the same pasture, they typically do not interact with each other.
Woodroffe and her colleagues looked at 421 cows and 54 badgers on dairy and beef farms at four sites in Cornwall between May 2013 and August 2015. Their unique experiment involved tracking the badgers and cattle and fitting the badgers with ultra-high frequency contact collars that could detect the collars worn by the cattle at distances less than two metres. In other words, any time a collared badger got within six feet of one of the collared cows, the badgers’ collar would log it. The data show that of the GPS locations falling on the farms, almost 60% were in cattle pasture and that, statistically-speaking, badgers actively chose this type of habitat. Despite this obvious preference for cattle fields, however, in almost 3,000 nights when the contact collars were in use there were no direct contacts between the two species. Furthermore, when you combine the GPS and contact collar data it shows that in just over 8,000 nights of monitoring when badgers were located in the ranges of cattle, and therefore could potentially have wandered up to each other, there were no occasions when a badger was detected within 1.5m of a cow.
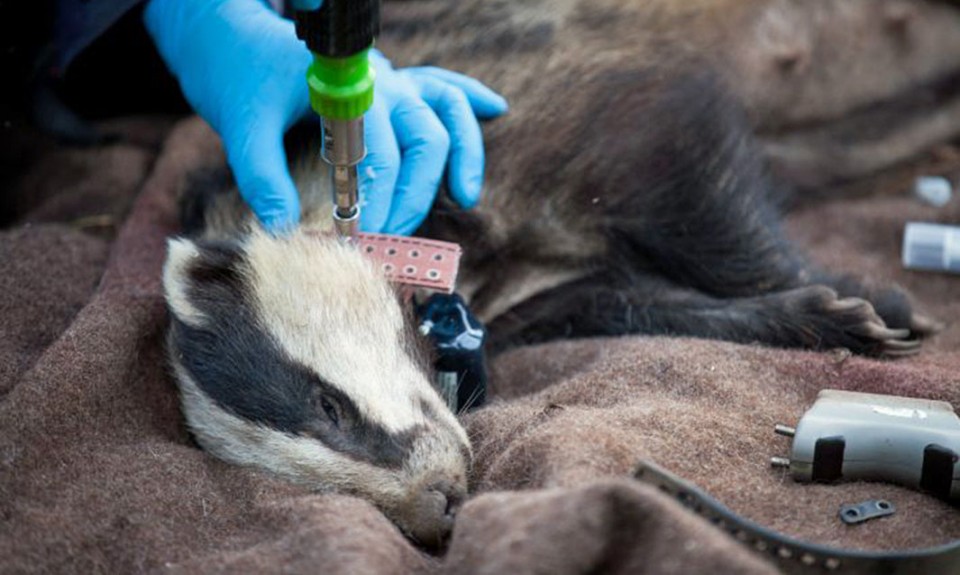
The researchers are quick to point out that absence of evidence is not evidence of absence, so we can’t say badgers and cattle never come together, but their findings imply that such occurrences are rare and that direct airborne transmission of TB between badgers and cattle is unlikely to be a significant pathway of infection. Instead, these data imply we should be looking more closely at environmental contamination as a major pathway. The data presented by Woodroffe and her team tie in with those collected by Ariane Payne at the French hunting and wildlife agency and colleagues. Payne and her co-workers studied wildlife interactions with livestock on 25 farms in the Côte d’Or area of north-eastern France and, in their paper to the European Journal of Wildlife Research in 2016 wrote:
“We observed a single direct contact in a pasture: the badger came close to the manger, a cow approached the badger in an investigative manner from the other side of the fence and the badger quickly ran away.”
Payne and her colleagues concluded:
“Our findings suggest that biosecurity measures should primarily target pastures, where priority actions should be designed to limit the attractiveness and access to water points for wild boar and to feeding troughs for badgers, whereas measures concerning salt licks should target red deer and wild boar.”
The science world seems broadly divided on this issue and most appraisals I have come across fall clearly into one ‘camp’ or the other. In 2007, the UK government’s then Chief Scientific Officer Sir David King wrote that “Badgers are a clear source of infection for cattle” in his report on Bovine TB to the Secretary of State – many consider this is still the case a decade on, although the pathway(s) and frequency of this transmission, if it does occur, has yet to be firmly established. Former government TB committee member Martin Hancox argues, however, that bTB is a disease of cattle, not of badgers, and is not self-sustaining; in other words, bTB doesn’t persist in the badger population without infected cows present to continually ‘top it up’. In Hancox’s view the insensitivity of the current tests for bTB results in “a huge hidden reservoir” of bTB in cattle that is transmitted within the herd to sustain infection. Badgers are, according to Hancox and others, ‘scapegoats’ for a lack of proper testing and biosecurity on farms.
Are badgers the only potential transmitter of bTB?
Short Answer: No, various mammals are known to be susceptible to the bacteria, including foxes, deer and rats. Most of these are, however, “spill-over hosts” (i.e. the disease kills them before they become infectious) and badgers are the only confirmed “maintenance host” (i.e. an animal that can carry the disease and shed it into its surroundings). Elsewhere, red deer and wild boar are maintenance hosts (not in Britain), while roe deer have also been implicated, but more data are needed.
The Details: No. Bovine tuberculosis has been recorded in a variety of wildlife, but it is important to understand the difference between a maintenance host and a spill-over host. In a maintenance host the infection is chronic, often contracted at an early age and the infectious bacilli are shed by animals of any age. Infection is endemic among maintenance hosts and usually passed between members of the species by direct contact. Maintenance hosts can sometimes pass the disease to other species, but the higher mortality means that these individuals either die before they reach an infectious (bacteria-shedding) stage, or recover from the disease – these are spill-over hosts. Within an environment, maintenance hosts maintain the presence of a disease, while spill-over hosts are essentially ‘accidental infections’ and the disease does not persist in their populations.
Martin Hancox argues that cattle are the only maintenance host for bTB and that the disease dies out in badgers without cattle there to top it up (i.e. that badgers are actually spill-over hosts), but many scientists consider the progression of the disease in badgers makes them a maintenance host. Badgers certainly seem to have a remarkable tolerance to the bacterium and generally between 50% and 80% of tuberculous individuals have no observable lesions. Moreover, a paper published in The Veterinary Record during 1998 reported that infected badgers generally have few sites of infection and small numbers of the bacilli in their tissues.

A review of M. bovis infection in wild mammals from the UK, headed by Central Science Laboratory biologist Richard Delahay, listed ten species other than badgers from which this bacterium has been isolated. Included in this list are the fallow deer (Dama dama), red deer (Cervus elaphus), red fox (Vulpes vulpes), mink (Mustela vision), mole (Talpa europaea), brown rat (Rattus norvegicus), ferret (Mustela furo) and domestic cats (Felis domesticus). A recent study of 1,307 bank voles (Myodes glareolus) found that only one yielded positive results for M. bovis, leading the authors to suggest that this species is relatively unimportant as a reservoir for bTB. Similar studies have shown that rabbits and mice can be experimentally infected with bTB, although no records are known from the wild. Most of these species are thought to be spill-over hosts in Britain, although the situation is unclear in deer and wild boar (Sus scrofa).
Wild boar and red deer are considered maintenance hosts for bTB in Europe, with geneotype mapping studies having suggested a link between the epidemiology of TB in domestic livestock and wild ungulates in southern Spain. More recently, work by Sébastien Lambert at the National Hunting and Wildlife Agency in France has suggested that infected roe deer (Capreolus capreolus) develop lesions that would allow excretion of bTB bacilli, making them a potential spill-over host. In Britain, there are occasional reports of bTB in park or farmed deer; one in Yorkshire during January 2012 identified 35 (3.7%) infected animals in a herd of 953.
We know very little about the situation in wild deer populations. A survey by Defra and the Deer Initiative found that the incidence of bTB in deer was generally low (less than 1%) in England, although in a single cluster in south-west England prevalence reached 15%. The survey identified that fallow were the most likely species to be infected, although this may also reflect their being our most numerous deer species. In their 2008 advice document on tuberculosis in deer, the Deer Initiative note:
“The indication is that, under current conditions, the majority of infected wild deer populations (in Southwest England and Wales) are most likely to be acting as spill-over hosts of M. bovis, reflecting the presence of bTB in an area rather than acting as a reservoir of the disease for cattle. They are more likely to pass bTB on to other deer, rather than posing a significant risk to livestock, but any infected animal of any species might shed infection and again, the risk is increased when deer are present at very high densities.”
In the UK, the Tuberculosis (Deer) Order 1989 requires any suspicion of TB in deer (farmed, park or wild) be reported to the Duty Vet at the local Animal Health Office, while the Tuberculosis (Deer) Notice of Intended Slaughter and Compensation Order (1989) mandates reporting of TB in farmed deer. There is, however, no routine statutory bTB testing programme for deer in Britain, and skin testing must be undertaken at the owner’s expense, which may hamper dealing with infections where they arise. Indeed, in their paper to Veterinary Record documenting the outbreak in farmed red deer in Yorkshire in 2012, a team led by F. Busch at the Animal and Plant Health Agency noted:
“The concern over the effectiveness of the SICCT alone, and the fact that any BTB testing in deer in England is carried out at the owner’s expense complicated the management of this BTB incident.”

Wild boar are considered the most important wildlife maintenance host in parts of the Mediterranean, particularly Portugal and Spain, with surveys culturing M. bovis from about 50% of boar tested. In Europe, however, boar populations are artificially managed for hunting, provisioned with supplemental feeding for example, which may increase local densities. The UK wild boar population is relatively small and localised and, as such, it’s not currently considered a maintenance host here. Nonetheless, the discovery of a young female boar with lesions consistent with bTB infection in Ross-on-Wye in 2010 suggests this is a situation worth monitoring, particularly as the UK population appears to be increasing in both size and distribution and boar are well known to visit farms on the continent. Ariane Payne at the French hunting and wildlife agency and colleagues used camera traps to study visits to cattle feeders by red deer, wild boar and badgers on 25 farms in the Côte d’Or area of north-eastern France; of the 236 visits recorded, most were by wild boar (142 or 60%), with badgers visiting about one-third as often (51 visits, 22%).
It has been suggested that the M. bovis bacteria may be transmitted on the feet of birds or could hitch a ride on an invertebrate, and that this may promote the spread of the disease. To the best of my knowledge, however, this remains a theoretical risk and has never been demonstrated.
Why don’t we just vaccinate cows against TB?
Short Answer: Vaccination is expensive as is the only test (the DIVA test) that can tell the difference between “reactors” that are vaccinated and those that are genuinely infected. Without this ability to discriminate a herd cannot be certified as TB-free. There is also no provision in existing EU legislation for a status of “vaccinated but TB-free”.

The Details: This is probably the most common question I have come across and it’s a good one. After all, vaccination is the standard method by which we go about protecting people and animals from infectious diseases. The problem, in this case, is that famers are not legally allowed to vaccinate their cattle, because the vaccine makes it hard to tell whether the cow is genuinely infected with bTB.
The European Union (EU) allows free movement of milk and dairy products within its member states provided certain hygiene standards are met. As we’ve covered above, cattle are tested for bTB using the tuberculin skin test, but vaccination with the only available vaccine (the Bacille Calmette-Guerin, or BCG) interferes with the skin test (SICCT), making it difficult to tell infected cows from vaccinated ones for at least six months post-vaccination. There is a test, referred to as the DIVA test (Differentiate Infected from Vaccinated Animals), that can tell the difference between infected and vaccinated cattle, but it is expensive and not widely available. Furthermore, there is no provision in EU Directive 78/52/EEC for certifying products as being from ‘TB-free but vaccinated herds’. In essence, vaccinating the cows means that the UK would lose its ability to trade cow products with other EU countries; in 2011, trade in live cattle, meat and dairy products amounted to some £1.7 billion. The UK government would require a dispensation from the EC in order to initiate vaccine trials and, if such trials were successful, member states would have to vote to repeal the directive. It remains to be seen what will happen in post-Brexit Britain.
There are currently no field data from the UK, for the reasons discussed, to show how effective BCG vaccination of cattle would be in eradicating the disease, but small-scale studies from Ethiopia and Mexico suggest vaccination is up to 68% effective. Similarly, in a their review published in Comparative Immunology, Microbiology and Infectious Diseases in 2012, Nazneen Siddiqui and colleagues at the Institute for Animal Health note that a number of studies have demonstrated how vaccination of newborn calves with the BCG vaccine confers a significant degree of protection and point to this being a widely-used strategy for controlling TB in humans. Any vaccination would, however, provide a spectrum of protection, with some cattle being fully protected, some experiencing reduced disease, and some that derive no protection at all.
(For those interested in reading more, I recommend the authoritative review published by Professor Mark Chambers and colleagues in Veterinary Record during July 2014, which is available free online.)
Can’t we vaccinate badgers, instead?
Short Answer: Yes, and trials have been underway for several years. The problem, though, is that vaccination doesn’t cure infected badgers (that’s not how vaccines work) and any vaccine is likely to convey a spectrum of protection against future infection. If badgers are responsible for sustaining TB in cattle it seems likely some combination of vaccination and culling of infectious animals would be required, in the short-term at least. Recently, media reports suggested vaccination disrupts badger social structures and helps spread the disease, but this has since been shown not to be the case.
The Details: An injectable BCG for use on badgers (appropriately named BadgerBCG) was licensed in March 2010 and has been used by several animal welfare charities as part of various badger vaccination trials in England and Wales. (BadgerBCG uses the Danish 1331 strain; the same used in human BCG immunizations in Europe.) There are, as far as I know, no published data from the field yet to show what, if any, impact vaccinating wild badgers has on bTB incidences. Five of six planned vaccination trials were cancelled by the UK’s former coalition government and several schemes were postponed for part of 2015 and 2016 owing to a worldwide shortage in the vaccine. Nonetheless, an article in Farmers Weekly in February 2017 described some broadly positive early results from a large-scale field trial of badger vaccination in County Kilkenny, Ireland. Furthermore, in an article to the Irish Independent during the same month, the Minister for Agriculture, Food and the Marine, Michael Creed, was quoted as saying the results of the trial were “very positive”, demonstrating that the oral vaccination of badgers has a significant protective effect in badgers under natural conditions. Further trials are currently underway in Ireland, with preliminary results due in 2018.

Studies on captive badgers have resulted in a lot of very misleading headlines and suggestions that vaccination is somehow a ‘magic bullet’ to the problem of bTB. One recent study, for example, was widely reported in the media as showing that the BadgerBCG reduced the incidence of bTB in badgers by 74%. In fact, the study found that, using one particular serological test, only 4.5% of vaccinated badgers responded to challenge with the bacteria, compared with 17% of non-vaccinated animals. This is not the same as saying the vaccine protects 74% of badgers; it simply shows that, among this group of 262 badgers, there was a 74% reduction in one serological response to bTB challenge. Overall, the study suggests that there is a benefit to the vaccine, but it could not say how big the benefit was.
Generally speaking, vaccination studies on captive badgers show a moderate level of protection, with vaccinated individuals typically living longer than non-vaccinated animals while experiencing a slower progression of the disease, fewer bacteria in the lungs, fewer tissue lesions and, consequently, reduced shedding of bacteria. In none of the trials has vaccination cured the badgers of TB; but this should not be expected, because this is not how vaccines work. Indeed, many have pointed out that vaccination will do nothing to help already infected badgers, which is correct because vaccination is aimed at conferring immunity to subsequent exposure.
Any given vaccine, as mentioned for cattle above, is also likely to confer a spectrum of benefits according to the individual. Nonetheless, a vaccine doesn’t have to be 100% effective and you don’t need to vaccinate 100% of animals. Instead the vaccine needs to be sufficiently protective, and sufficiently widespread in the population to slow the progression of the disease such that it dies out; this concept is known as herd immunity. One study on captive badger cubs, for example, found that the chances of an unvaccinated cub testing positive for TB was only 21% when at least one-third of the social group had been vaccinated.
Briefly, recent work by a team led by Andrew Robertson at the University of Exeter assessed the uptake of oral baits, laced with the Bacillus Calmette-Guerin (BCG) vaccine, by badgers and non-target species in Gloucestershire over 10 nights in August 2013. The study showed that baiting with the vaccine was plausible, but that enclosing the baits in packaging was the best option to discourage non-target species (mainly rats and mice) from taking them. Robertson and his colleagues also observed that badgers were initially hesitant at taking the baits and suggest that some 'pre-feeding' may be needed first to overcome this neophobia. Previous studies have demonstrated that the BCG itself can be encased in a lipid base to protect it from being destroyed by the badger’s stomach secretions.

Owing to the lack of an appropriate cure for the disease in badgers, there has been a large movement supporting a cull of badgers to remove the ‘high proportion of sick badgers in TB hotspots’ that would continue to spread the disease, followed by a proactive vaccination campaign to slow any resurgence of the disease.
The problem, of course, is that trapping, testing and then shooting infected badgers is very labour intensive and expensive, so strategies more typically aim to simply reduce the badger population in the area, culling healthy and infectious individuals alike and this makes it difficult to quantify the impact the cull is having on TB prevalence. Furthermore, there has been some suggestion that such indiscriminate culling may exacerbate the situation because, as it doesn’t target infected animals, it may increase the number of infected relative to healthy animals in the population. There are tests available that can detect TB infection from badger droppings and could potentially be used to analyse latrines to identify infected clans for removal. Within the hotspot areas infection rates are generally considered to be around 20% (i.e. 1 in 5 badgers are infected with bTB).
Finally, in 2017, an article in the Daily Mail claimed that vaccination may even exacerbate the spread of the disease in the wild, disrupting the badger social group and causing them to move away, taking the infection with them. This does not, however, appear to be the case.
A recent study by a team led by Zoological Society of London biologist Rosie Woodroffe found that vaccinating badgers, which doesn’t remove them from the group as culling does but instead confines them to a cage trap for a night, doesn’t disturb the social structure of the clan. The team analysed movement data from 54 GPS-collared badgers, 39 vaccinated and 15 un-vaccinated, at four sites in Cornwall to see what effect restraining them in a trap overnight and/or vaccinating them had on their ranging behaviour. The data show that BCG vaccination had no significant effect on the badgers’ monthly home range size. Vaccination didn’t impact the distance moved per night, or the likelihood that the badger would trespass into a neighbouring territory. Furthermore, the trapping itself, whether it involved vaccination or not, didn’t affect ranging behaviour either. Writing in the Journal of Applied Ecology in 2017, Woodroffe and her colleagues concluded:
“In contrast with culling, live trapping and vaccinating badgers did not measurably alter their movement behaviour, fuelling optimism that vaccination might contribute positively to cattle tuberculosis control.”
Are DEFRA culling badgers at the moment?
Short Answer: Yes. Licences have been issued to cull badgers in parts of Devon, Wiltshire, Somerset, Dorset and Cheshire.
The Details: In September 2012, Natural England issued a 21-condition licence, under the Protection of Badgers Act (1992), for a trial cull of badgers in western Gloucestershire; the following month they issued a second licence, for a trial cull of badgers in western Somerset. This was the start of the government’s 25-year-strategy to eradicate the disease in Britain and the plan was for badgers to be ‘free shot’ (i.e. free-ranging badgers attracted to bait stations), or cage-trapped and shot, at night by trained marksmen using high-powered silenced rifles over a six-week period.

The licences permitted culling of badgers during the open season each year for four years with the ultimate goal being to reduce the badger population in each of the cull zones by 70%; based on earlier sett surveys conducted by DEFRA, this equated to removing around 5,000 badgers. Animal welfare groups immediately raised concerns about the possibility that attempts to shoot low slung, muscular animals in the dark would lead to many badgers being wounded rather than killed outright. Monitoring reports by Natural England leaked to some of the press in February 2014 suggested that one-third of the badgers were shot in the wrong part of the body and one-fifth had to be shot a second time.
Contrary to popular misconception, the 2012 trial culls were not aimed at assessing whether culling badgers reduces incidence of TB in cattle. (As far as DEFRA are concerned, it does and this isn’t a matter for debate.) Instead, the culls were designed to assess how suitable such ‘free shooting’ is -- in terms of its practicality, safety and humaneness -- as a method of culling wild rural badgers at night. Some have questioned why only around 190 of the 5,000-or-so badgers that were due to be shot were assessed for humaneness of the kill.
A bigger problem for many, myself included, was that none of the carcasses were tested for bTB; so a vast potential dataset of infection was lost and we gained no insight into how widespread TB infection is among these ‘hotspot’ badgers, nor how strains of TB are distributed among the badger population. DEFRA have said they see no need to test the badgers because tests are unreliable and they already have data on infection rates, while sceptics suggest that DEFRA don’t want to test the badgers only to find that fewer than expected were infected.
Widescale interference from badger protection groups resulted in significant disruption to the culls and caused many areas to miss their quota by a considerable margin. More recently, official figures suggest that targets are now being met, although, speaking ahead of the Bovine TB Conference held in London during March 2017, the ZSL’s Rosie Woodroffe accused Defra of changing the way it assesses the number of badgers in a cull area and how it sets the target for the minimum number to be killed to ensure that its culls appear successful. In an interview with BBC News, Professor Woodroffe is quoted as saying:
“Where few badgers were being killed, they lowered the targets; where a lot were killed, they raised them. This means that there is really no way to tell what reduction in badger numbers was achieved by these culls. Culling that was consistently ineffective would look like a low badger density and prompt a reduced target…”
Nonetheless, in August 2016 the government issued licences for culls to be carried out in several additional areas of Britain, including Herefordshire, Cornwall and Devon, bringing the total number of counties culling badgers to 10.
The UK signed up to the Bern Convention, a legally binding international agreement by which member states agree to conserve and protect their wild flora and fauna in their natural environments, in 1982. A complaint was submitted by wildlife charities who suggested that the UK government were in breach of the conditions of the Bern Convention by giving the ‘green light’ to a cull. In September 2011, however, the Bern Bureau Standing Committee met and decided that the proposed cull “should not cause a threat to the [badger] population if the monitoring is carried out properly.”
Culling badgers in Ireland has reduced their incidences of TB in cattle, right?

Short Answer: Northern Ireland (NI) has not implemented a badger cull in the same manner as England, although they have recently begun euthanising TB-positive badgers trapped during their vaccination programme instead. The Republic of Ireland (RoI) has operated a badger cull, but these have been much more substantial and widespread than those in England with badgers completely removed from some areas. Indeed, sustained removal of badgers was shown to reduce herd breakdowns and prevent new cases over the duration of the study, but has failed to eradicate the disease completely.
Widespread reduction in herd breakdowns were seen in Ireland following the implementation of a test-and-slaughter policy in 1950; this reduction was sustained in the RoI (currently herd infection is about 5%), but not in NI. NI saw a significant increase in bTB cases that peaked in the early 2000s before dropping again to around 5% of the herds infected in 2005. In recent years the situation has deteriorated and currently (late 2017) about 10% of herds are positive for TB.
In both NI and RoI more resource has been directed at educating key stakeholders in farming and livestock management, improving biosecurity, cattle testing and post-slaughter surveillance than in England and Wales. Additionally, landscape use in Ireland is different to England, as is badger sociality and population density, which significantly complicates direct comparison. In 2016 it was announced that the RoI cull would end and a vaccination trial would begin.
The Details: It is estimated that around 40% of British cattle were infected with TB during the first half of the 20th century, but the introduction of a widespread test-and-slaughter policy in 1950 led to a rapid reduction, such that less than 0.3% of cattle tested positive in the 1970s. In Northern Ireland, only 0.06% of tested cattle were found to have TB by 1986.
In the late 80s the situation changed; the incidence began to rise, as it did in England and Wales – slowly at first, but gaining pace during the late 1990s until about 10% of herds were infected by 2003. A steep drop of reactors followed, reaching a minimum of about 5% in 2007, where it stayed until 2011, after which the situation deteriorated and more herds began testing positive. Statistics from the Department of Agriculture Environment & Rural Affairs, Northern Ireland show an increase in infection in recent years, with almost 10% of herds testing positive at the end of 2017.

Of particular interest with the situation in Northern Ireland is that the decline in herd infection after 2003 wasn’t associated with a cull of badgers; there hadn’t been one. Many authors suggest that the launch of the Enhanced TB Eradication Programme in 1992 and its subsequent review in 2002 deserve credit for the reduction in reactive herds. The programme saw improvements in stock management and additional TB-control measures, including tightening up testing, an increase in the number of vets available to carry out the testing and increased subsidies provided to farmers to encourage the use of fencing to keep cattle and badgers apart.
That the progress in reducing TB infection had stalled by 2007/2008 led to calls for a cull of badgers to be undertaken. In 2013 a vaccination trial, whereby badgers were cage trapped and vaccinated against TB before being released, began in Northern Ireland and in July 2015 plans were announced to euthanise badgers caught during this trial that tested positive for TB. In September 2017, DAERA confirmed that there were still no plans for a badger cull in Northern Ireland and that the results of the vaccination field trial are anticipated in early 2019.
The situation in the Republic of Ireland is different again. When the RoI’s “Bovine TB Eradication Scheme” began in 1954, an estimated 80% of herds were infected and the same test-and-slaughter policy resulted in a significant decrease. Indeed, over a decade about 16% of the national herd was slaughtered and herd prevalence dropped to about 5%. Unlike NI, however, the RoI sustained this level and no significant increase in reactors was seen. The RoI made badger culling a significant part of its eradication programme and it has been claimed that this explains the continued low trend.

In 1989, a three-phase program was initiated to assess the impact of culling badgers on the incidence of TB in cattle. The trial, called the East Offaly Badger Research Project, saw the sustained culling of badgers from an area of central Ireland over a six-year period up to 1994. The TB incidence inside this cull zone was then compared with neighbouring areas where limited local culling was undertaken.
The results of the East Offay project were striking: by the end of the trial period all culling areas had seen a decrease in herd breakdowns, and the largest drop was seen in the sustained cull zone. By 1995, the incidence of TB in cattle was 86% lower in the sustained cull zone than in the RoI as a whole. Localised culling resulted in a more modest, but still statistically significant, reduction over the national average. Subsequently, the Four Areas Project was established and ran between 1997 and 2002, comparing cull and no-cull areas and found similar reductions in TB incidence in response to badger removal (50-75% reductions), although the study implementation was more controversial, with several anomalies that complicate statistical interpretation. Nonetheless, both trials suggest badger culling can significantly reduce herd breakdowns in Ireland.
The results from the RoI, which have been in stark contrast to those of the RBCT in England, supported calls for increased badger culling in the Britain, but it’s not a straightforward generalisation to make. There are differences in farming practices, badger social structure, population density and land configuration between England and the RoI, which complicate comparison. The data also suggest that breakdowns can only potentially be stopped if badgers are completely removed from the area and prevented from returning, which is more logistically challenging and less palatable to the public in England than it appears to be in Ireland. Furthermore, as Tim Roper puts it in his excellent summary of the issue in Badgers:
“… the explanation may simply be that badgers cause a higher proportion of cattle TB incidents in Ireland than they do in Britain.”
Even taking the data from these trials at face value, the situation is not straightforward and the number of RoI reactors increased 13% between 2006 and 2007, despite continued culling of badgers. Indeed, a 2015 paper to Veterinary Microbiology Simon More at the University College Dublin and Margaret Good at the Department of Agriculture described examples where cattle infection appeared to decline in response to badger culling in Ireland along with instances where culling appeared to have had little or no impact. The RoI are also further advanced in their research into badger vaccination than England or Wales, with a large-scale vaccination programme due to release preliminary data next year (2018).

Finally, it has been argued that the frequent rigorous testing and slaughter of infected cattle, combined with more stringent biosecurity better explains the fall in TB infection rates in Ireland, and that the badger culling was irrelevant to the situation. This is a difficult position to argue, but, in August 2016 the Irish government announced plans to phase out culling in favour of a vaccination programme. Preliminary results of this study, led by Inma Aznar at University College Dublin, were published in Preventative Veterinary Medicine in January 2018 and suggest vaccination has potential to reduce TB infection in badgers in Ireland.
What’s the big deal with culling badgers?
Short Answer: There’s no conclusive evidence that such an invasive, expensive, publicly unpopular and, according to some, inhumane practice reduces incidences of bTB infection. Initially, we also thought culling disrupted the social structure of badgers, causing them to emigrate and take the disease with them (thereby making the situation worse), but this so-called “Perturbation Hypothesis” has been largely debunked now. There are, however, some data suggesting that reactive culling (i.e. that in response to a breakdown) increases the chance of a breakdown on neighbouring farms.
The Details: The consensus among wildlife biologists and many vets is that killing badgers is an expensive waste of time and a response to bTB that is not supported by the science. Evidence from culling trials suggests that localised culling of badgers can only achieve modest (i.e. 16-20%) declines in cattle TB rates when 70% or more of the population is removed.
This culling strategy is extremely expensive, labour intensive and, perhaps more importantly, grossly unpopular with the public, particularly animal welfare groups; the result is that culling operations are often subjected to protests and interference, which adds additional policing costs. Furthermore, culling isn’t selective, so both healthy and infected animals are removed, which some have suggested may increase the number of infected animals in the group if a higher proportion of uninfected animals end up being removed.

Nonetheless, in a paper to PLOS One in 2016, Graham Smith and colleagues presented the results of their computer model comparing the difference selective and un-selective culling of badgers made to bTB infection rates. While just over 80% fewer badgers were killed if selective culling was employed, their model found this had no obvious impact on the number of herd breakdowns when compared with non-selective culling or non-selective vaccination and selective culling was unlikely to be justifiable based on the extra cost of testing badgers first. The NFU argue that culling does have an impact on bTB rates and, on their TB-free England website they state:
“The alternative to a cull would either be to do nothing and wait, quite possibly for ten years, for another solution, by which time the incidence of the disease would be even more severe and take even longer to control, or to vaccinate badgers in heavily infected areas. This would be very ineffective and costly and there is absolutely no research data to indicate what the effect on the incidence of bTB in cattle would be.”
In recent years, some have argued that culling badgers is a distraction from tackling the real problem of inefficient testing of cattle and poor biosecurity on farms – the money could, in their opinion, be better spent on developing more sensitive tests for cattle and vaccines for both cattle and badgers.

There is no consistent relationship between culling badgers and TB rates (i.e. a consistent cull, year-on-year, doesn’t result in a consistent decline in reactors or breakdowns) and this observation, Martin Hancox has argued, indicates that any reactor declines during badger culling schemes is actually no more than a coincidence; that it’s the weeding out of previously undetected infected cattle with subsequent rounds of testing that causes the decline. Certainly, unidentified infectious animals have a significant role to play and the computer model presented by Ellen Brooks-Pollock, Gareth Roberts and Matt Keeling in their paper to Nature in 2014 suggested cattle movement was responsible for the majority (84%) of newly infected farms. (A caveat here is that computer modelling is hampered by a lack of data on transmission routes and rates.)
The first attempt to assess the impact of culling badgers on rates of bTB took the form of the Randomised Badger Culling Trial, or RBCT (sometimes also called Krebs Trial), that ran between 1998 and 2007. The experiment looked at culled and control sites in 30 areas of England with a high bTB prevalence and one finding was that while culling badgers reduced TB incidences within the cull zone, it increased incidences in neighbouring areas. This scenario was dubbed the “perturbation effect” and was believed to have been caused by infectious badgers, disturbed by the culling operation, moving out of the area and infecting nearby farms/badgers outside the cull zone. The perturbation effect is still a widely-cited reason for not culling badgers, despite considerable criticism. In his report to DEFRA in July 2007, Chief Veterinary Officer of the day, Sir David King, wrote:
“Nevertheless, there was evidence of some detrimental effect outside the removal area and that removal disrupts badgers’ social groups. The ISG put forward the perturbation theory to explain these effects. Although we were not fully persuaded by it, in the absence of any other theory, the perturbation theory was seen as a plausible explanation.”
Martin Hancox is uncompromising in his disagreement with the perturbation hypothesis; in his article in The Ecologist he considers it a ‘gaffe that slipped past peer reviewers’, writing that:
“The whole perturbation hypothesis is based on very ‘Dodgy data and bad science’.”
The question is not whether culling induces some kind of social perturbation in badger clans; Stephen Carter and colleagues demonstrated that this happens and can remain for up to eight years in a paper to the Proceedings of the Royal Society in 2007. Instead, the question is whether this perturbation can actually have a meaningful effect on the number of bTB reactors, particularly given that only a small percentage of the badger population appears to be infectious. Consensus is growing that it does not; but this is not universal. An interesting review by David Wright at the Queen’s University Belfast and colleagues, published in Scientific Reports during 2015, for example, suggested that badger persecution may contribute towards sustaining bTB hotspots. They concluded:
“In particular, this is the first study to highlight the potential importance of badger population disturbance other than officially sanctioned culling in sustaining the bTB epidemic. Persecution did not appear to substantially reduce bTB risk and may have exacerbated the problem by triggering perturbation.”

More recently, in a paper to PLOS One published in 2016, Jon Bielby at the Zoological Society of London and colleagues reported that reactive culling of badgers (i.e. culling them when TB was detected, rather than as part of a pre-emptive plan) significantly increased the chances of a herd breakdown on neighbouring farms between one and five kilometres (0.6-3 miles) away.
In the end, the big issue is that you have to kill a lot of badgers, many of which are not infected with the disease, for a modest, if any, decline in reactor rates. When you start culling it tends to be fairly straightforward, but as the programme continues you’re left with progressively fewer and fewer badgers, now much warier than at the start of the operation, and this increases both the difficulty and expense of the cull. Furthermore, culling can never offer a long-term solution because it must be regular, arguably continual, in order to account for populations recovering and badgers immigrating into the area.
Hasn’t the badger population exploded in the last decade? Surely, without any predators, their numbers need controlling?
Short Answer: Possibly, although the cull is aimed at reducing bTB incidences and not “control badger populations”. Badger numbers have increased since their protection under the 1992 Badgers Act and there are calls to update this legislation to allow landowners the ability to manage populations on their land. Badger density and impact varies considerably with habitat, however, and no uniform “ideal population” can be applied. Any plan to reduce numbers must first establish how many is acceptable so a target might be set.
The Details: There have been many statements circulated of late stating how badger numbers have risen dramatically, thanks to legal protection granted in the early 1990s, to the point where the population is now ‘out of control’. There have been several attempts to estimate the national badger population and the most recent, published in 2017, suggests badger populations have increased. It should be noted, however, that requirements for wild mammal population management should be treated independently to calls to cull a particular species in response to a disease epidemic. So, just because the badger population has increased since it was afforded legal protection does not influence whether culling them is a legitimate way of controlling bTB.

Getting a handle on badger numbers is not a simple task because densities vary considerably with location and habitat type. Furthermore, directly counting them is challenging owing to their largely nocturnal lifestyle, with a significant amount of their time spent underground. In their The history, distribution, status and habitat requirements of the badger in Britain report to the Nature Conservancy Council in 1990, Penny Cresswell, Stephen Harris and Don Jeffries estimated the British badger population to have been about 250,000 during the late 1980s, although this was extrapolated from an average social group size of about six animals based on observations and trapping largely from southwest England, where we now know the badger population to be densest.
The IUCN note that, by the end of the 1990s the badger population had increased by some 77% from mid-1980s level, although there seems to be considerable uncertainty around this figure and, in 2005, the Joint Nature Conservation Committee published their mammal report, which estimated there to be 288,000 badgers in the UK. Furthermore, in a short paper to the journal Scientific Reports published in March 2017, a team of biologists led by Johanna Judge at the Animal and Plant Health Agency used a combination of mark-recapture and hair genotype data collected from 120 main setts across England and Wales between 2011 and 2014 to estimate the average social group size.
Clan size varied considerably with habitat type, from about three to nine animals per group, but the average social group was estimated to consist of about seven animals. Based on these data, Judge and her colleagues estimated that there are approximately 485,000 badgers in England and Wales, suggesting the population has probably doubled since the 1980s.
The question of whether the badger population has grown too large is a matter of opinion; badgers have long been considered a pest by some farmers, particularly where they have built large setts and caused damage to cereal crops. I have seen many calls for legal protection to be relaxed or revoked altogether so farmers can ‘control’ badgers on their land.
I have not seen any recent estimates, but a survey in 1997 put the cost of damage caused by badgers (including damage to roads, crops, agricultural land, etc.) at just shy of £26 million per year. Conversely, one wonders how much badger watching tours contribute to the British economy. The question, however, is how many is too many badgers? It’s very difficult to calibrate nuisance and one badger may be one too many if it’s causing you problems. In the end, we have a duty of care to our wildlife and balances must be struck at the local scale.
So what is the solution?
Short Answer: There isn’t one, yet. Removal of infected cattle from herds to prevent reinfection is one key goal, with establishing “herd immunity” among badgers another. A revision of EU legislation to allow vaccination of cattle would allow a big step forward in battling the disease. Whether culling badgers can have any meaningful impact on TB incidence remains to be seen, but many authorities are quick to point out that bTB is a disease of cattle and it is in cattle that the solution must be found.
The Details: The solution to the bovine TB epidemic is likely to be multifaceted. First and foremost, we need a TB test that is both highly sensitive and highly specific – this is critical in ensuring we’re able to remove all infected cattle from the herd, without killing any unnecessarily. We also need a cost effective and accurate test that tells vaccinated and infected animals apart; if we have this, we can look to repeal the EC directive that prevents UK farmers from vaccinating their cattle against bTB. We need to better understand the role played by wildlife on TB epidemiology so we can determine where the greatest risk of spillover or reinfection may occur and respond proactively.
In terms of the response, we need a cheap and effective vaccine that can be administered to badgers (possibly other wildlife). Ideally, such a vaccine would be deliverable in a bait, akin to the rabies vaccine. The strategy may also require the identification and removal of badger setts proven to be infected with M. bovis. This costs money, though; money that the government almost certainly doesn’t have. It also takes time, and this can be difficult to justify when farmers are being driven out of business by this disease. Some tough decisions must be made, and they must be based on the best evidence we have.

In the short term, at least, it would seem that more effort needs directing at regular intensive testing and removal of infected cattle, tighter biosecurity governing cattle movements, and more research towards how badgers and cattle can be kept apart in barns and the farmyard. Attention should also be directed to assessing the impact of farming intensity of bTB risk. A study by Bertina Winkler and Fiona Matthews at Exeter University, published in Biology Letters in 2015, found that TB risk was lower on farms that had a greater percentage of hedges in boundaries, that grazed cattle on fields that had been cut for silage or hay and had smaller herds (50 cattle or fewer). Conversely, growing maize (a favoured badger food) and feeding silage was linked to an increased risk in both beef and dairy herds. Essentially, the lower the farming intensity, the lower the chance of a herd suffering a bTB breakdown, with the presence of hedges seeming of particular importance.
In the end, many experts argue that culling badgers is not going to solve the bTB problem in cattle – it may have an impact, and we simply can’t quantify this at the moment, but only be the control of TB in cattle through better detection, faster reactor removal and much tighter biosecurity on farms will solve the problem. Logically, however, if the wildlife reservoir (particularly that in the badger population) is as significant as some maintain, it seems improbable that bTB in cattle can be eradicated without addressing infectious wildlife.
External Links
DEFRA Animal Health
DEFRA Tuberculosis in cattle
Gamma Interferon Blood Test
Impact of localized badger culling on tuberculosis incidence in British cattle (Nature abstract)
International Study Group Final Report on Bovine TB (PDF Document)
National Farmer’s Union Bovine Tuberculosis Positive and negative effects of widespread badger culling on tuberculosis in cattle (Nature abstract)
TB Knowledge Exchange (University of Exeter & NERC)
Tuberculin Skin Test
Tuberculosis Vaccine Trial (BBC News)
Update on Measures to Tackle Bovine TB (DEFRA)